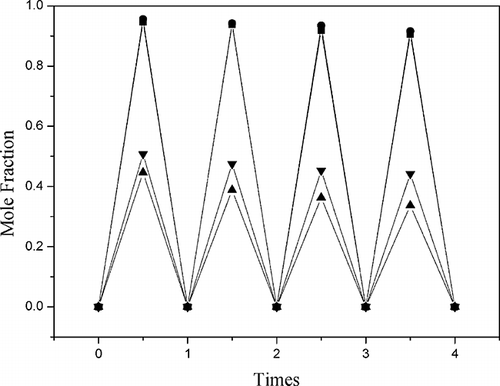
Figures & data
Figure 1. The mole fraction solubility (x) of NO as a function of temperature in different (2:1 mole ratio) ILs.
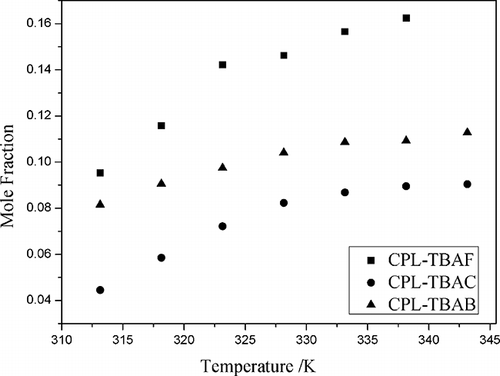
Figure 2. The mole fraction solubility (x) of NO2 as a function of temperature in different (2:1 mole ratio) ILs.
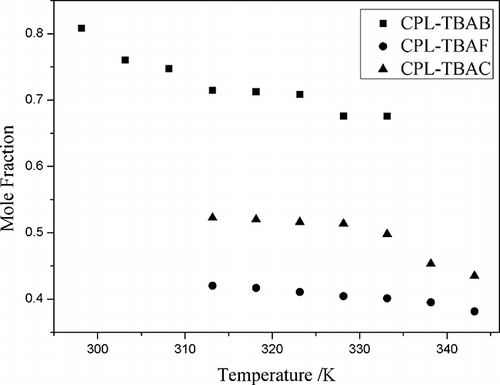
Figure 3. The color change of caprolactam tetrabutyl ammonium bromide ionic liquids with different concentrations of NOx. (a) Caprolactam tetrabutyl ammonium bromide ionic liquid; (b) after bubbling NO for 20 min; (c) after bubbling NO2 for 20 min.
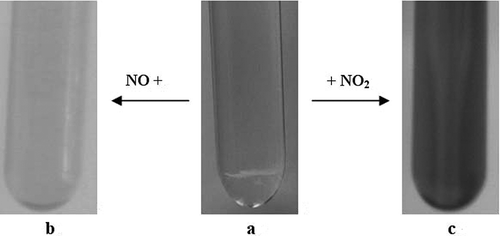
Figure 4. The mole fraction solubility (x) of NO as a function of temperature at different mole ratios of CPL and TBAB.
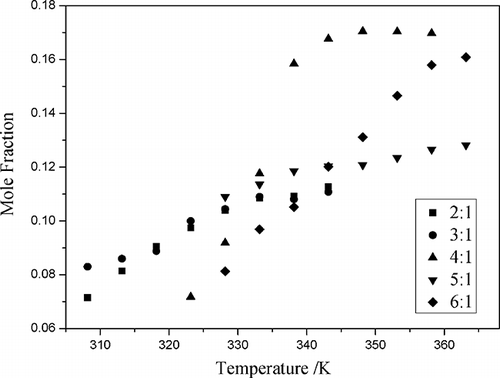
Figure 5. The mole fraction solubility (x) of NO2 as a function of temperature at different mole ratios of CPL and TBAB.
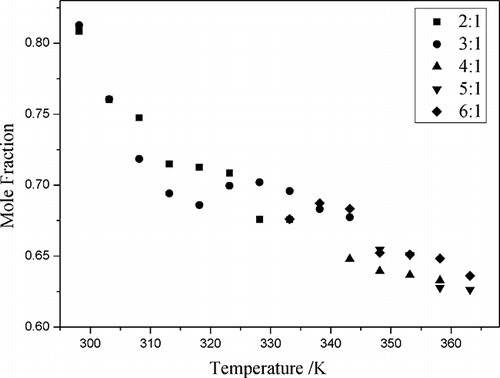
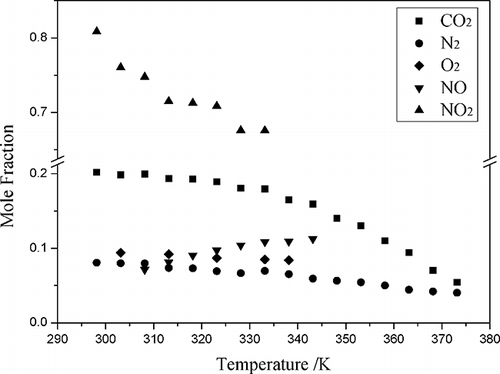
Figure 6. The mole fraction solubility (x) of N2, CO2, NO, and NO2 as a function of temperature at CPL and TBAB (mole ratio, 2:1).