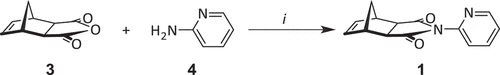Figures & data
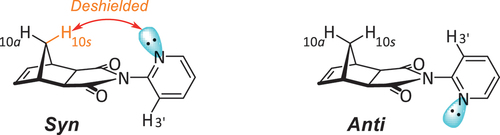
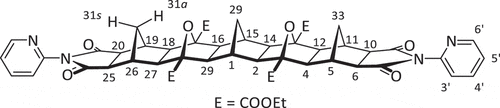
Figure 3. Structure of 2-pyridyl [5]polynorbornane 2. The left 2-pyridyl heterocycle is shown in the syn conformation, while the right is shown in the anti conformation.
![Figure 3. Structure of 2-pyridyl [5]polynorbornane 2. The left 2-pyridyl heterocycle is shown in the syn conformation, while the right is shown in the anti conformation.](/cms/asset/3508dac0-8f9a-4602-935d-bfdd012247f2/gsch_a_2384908_f0003_oc.jpg)
Scheme 2. Synthesis of the bis(2-pyridyl) framework 2. Reagents and conditions: (i) RuH2(CO)(PPh3)3, 100°C, DMF, 16 h, 46%; (ii) t-BuOOH, t-BuOK, −10°C – RT, THF, 16 h, 43%; (iii) 140°C, DMF, microwave, 30 min, 66%.
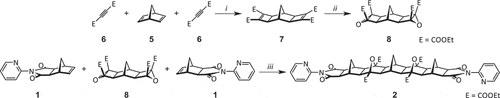
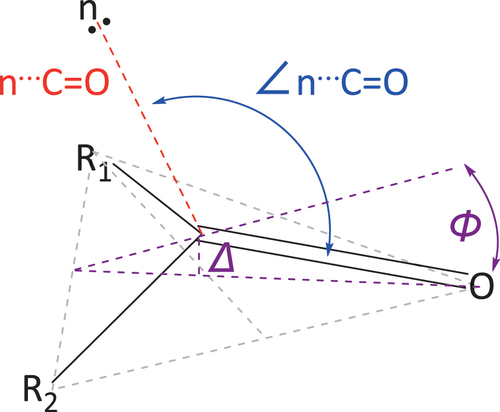
Figure 4. Calculated (a) transition state, (b) syn conformer and (c) anti conformer of imide 1, with annotated C3-N4-C2’-C3’ dihedral angles.
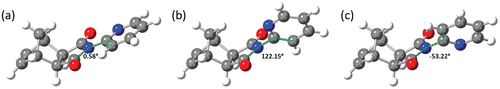
Table 1. Calculated (B3LYP/6-311 G* (298.15 K)) properties of conformers of imide 1.
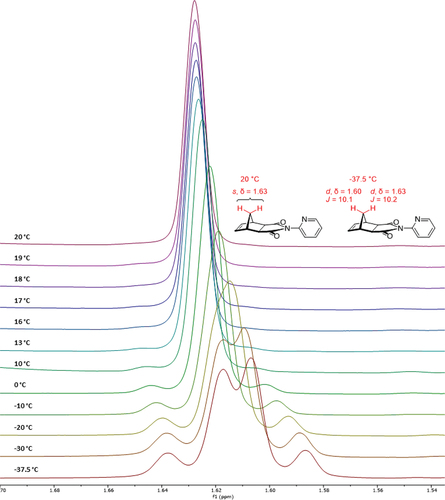
Figure 6. VT 1H NMR stackplot of 2-pyridyl [5]polynorbornane 2 (9.00–0.75 ppm), CDCl3, 500 MHz. Insets display the doublet assigned to the 31s and 33s protons.
![Figure 6. VT 1H NMR stackplot of 2-pyridyl [5]polynorbornane 2 (9.00–0.75 ppm), CDCl3, 500 MHz. Insets display the doublet assigned to the 31s and 33s protons.](/cms/asset/71a6dbb8-5c7d-482f-9637-95d7ee7f2e6f/gsch_a_2384908_f0006_oc.jpg)
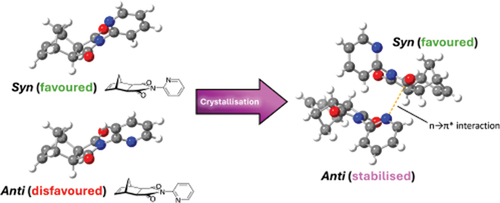
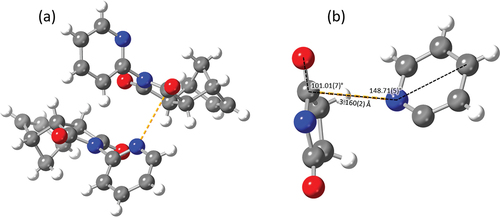
Figure 7. (a) Asymmetric unit of 2-pyridyl imide 1 highlighting the n→π* interaction (orange) and (b) annotated n→π* interaction of 2-pyridyl imide 1.
File not for review.docx
Download MS Word (762.2 KB)ESI.pdf
Download PDF (1.4 MB)Response to Reviewer Comments.docx
Download MS Word (181.6 KB)Data availability statement
Deposition number 2356985 in the Cambridge Crystallographic Data Centre (CCDC) contains the supplementary crystallographic data for this paper.