Figures & data
Figure 1. IFN-γ of different concentrations enhances the proliferation ability of hUC-MSCs.
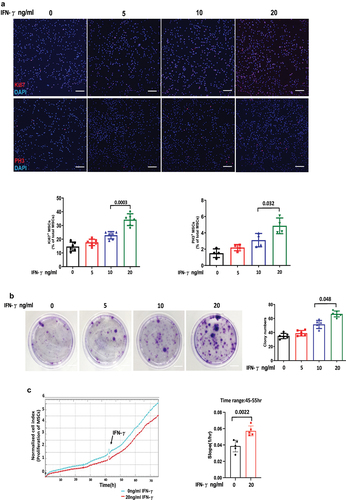
Figure 2. IFN-γ of different concentrations increases the migration ability of hUC-MSCs.
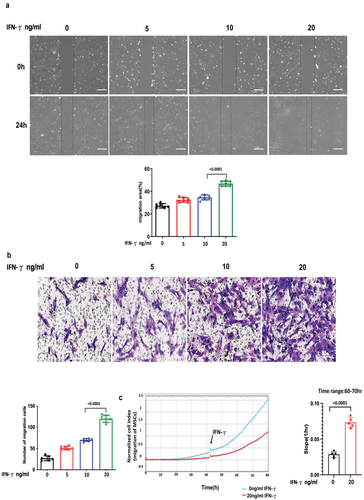
Figure 3. IFN-γ of different concentrations has minner effect on the stemness properties of hUC-MSCs as identified by induction of three-lineage differentiation and surface markers.
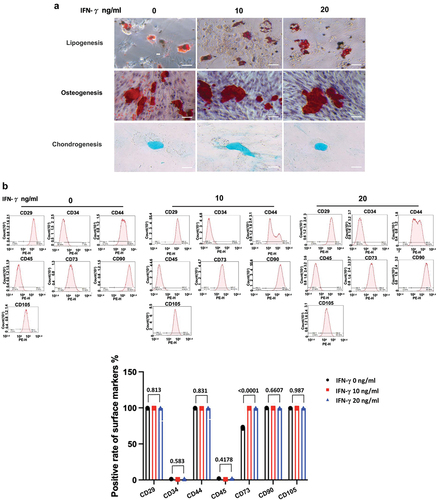
Figure 4. Loss of CD151 attenuates the effect of IFN-γ on the proliferation and migration of hUC-MSCs.
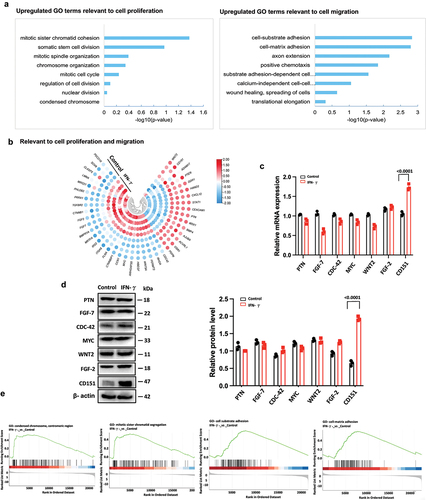
Figure 5. CD151 increases PI3K and AKT phosphorylation on promoters of proliferation/migration in hUC-MSCs.
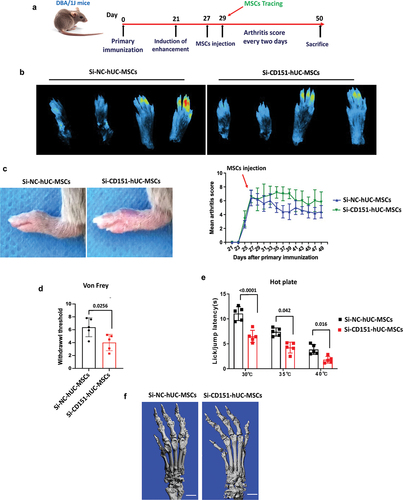
Figure 6. Knockdown of CD151 attenuates the local recruitment capacity and therapeutic effect of MSCs on CIA mice.