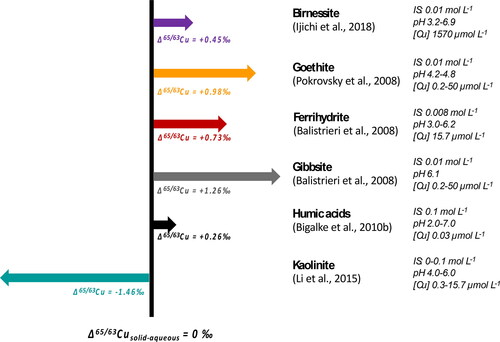
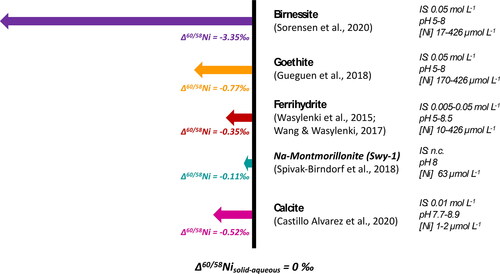
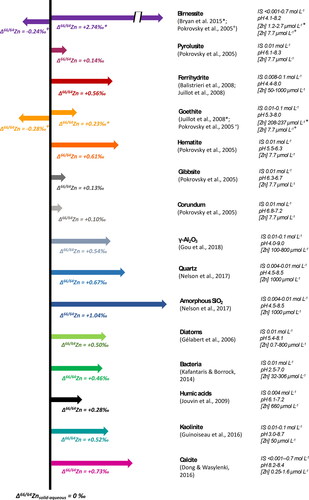
Figures & data
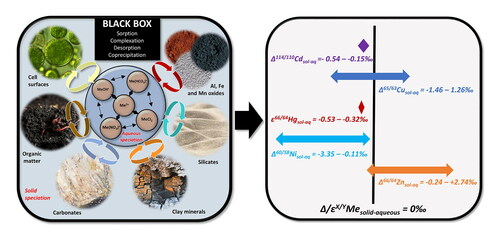
Figure 1. Schematic representation of the isotope fractionation “black box”. Stable metal isotope fractionation occurs in the environment due to various biogeochemical processes taking place during the redistribution of metals within and between different environmental compartments. The resulting isotope signature can result from sorption/desorption, complexation, evaporation/condensation, redox changes, interactions with plants and microorganisms, etc. This review focuses on interactions of Cu, Cd, Hg, Ni, Zn isotopes with environmentally relevant mineral and organic surfaces. During these interactions between the aqueous and solid phases, the resulting isotopic fractionation is driven both by the aqueous speciation of targeted metals and surface properties of the corresponding bearing phases. The distribution of the metals among different aqueous species itself may lead to isotopic fractionation in the solution, which may subsequently affect the fractionation during surface complexation. At the same time, the type and density of active surface sites influence the nature of the complexes and subsequently the isotope fractionation factor.
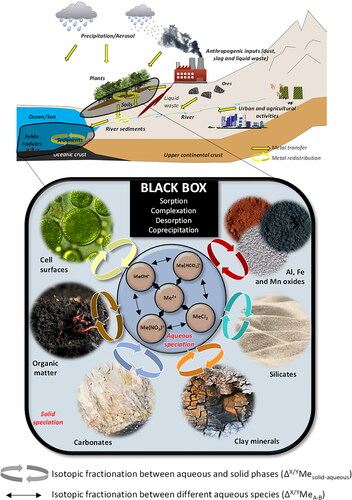
Figure 2. Maximum values of Δ114/110Cd after adsorption onto various surfaces (data from Wasylenki et al., Citation2014 were recalculated as Δ114/110Cd = Δ114/112Cd × 2).
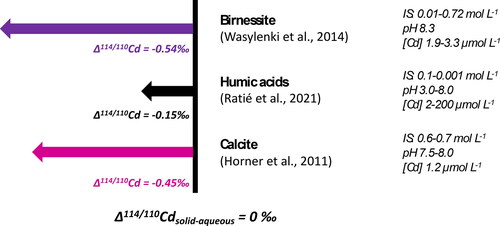
Figure 4. Mass dependent Hg isotope fractionation during sorption on different solid phases from solutions of different Hg chemical forms. The ε notation is used specifically for MDF of Hg isotopes (ε202/198Hgsolid-aq = δ202/198Hgsol – δ202/198Hgaq).
Table 1. Natural abundances (Rosman & Taylor, Citation1998) of selected metal isotopes and standard reference materials commonly used as isotopic reference points.