Figures & data
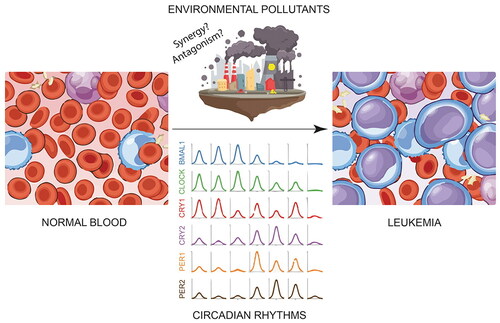
Figure 1. Distribution of the different types of leukemias. The percentages of the main types of leukemia are shown according to their acute or chronic character (A), the predominant cell type (B), their classification (C) and according to the ages at which they occur (D). The graphs are based on data taken from Elert (Citation2013).
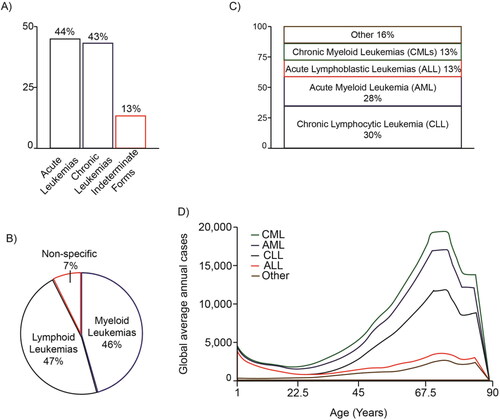
Figure 2. Mammalian circadian clock controls transcription and circadian oscillations of blood cell components. A) The mammalian circadian clock mechanism is a time-delayed transcription-translation feedback loop (TTFL). BMAL1-CLOCK constitute the positive arm and cryptochrome (CRY1 and CRY2) - period (PER1 and PER2) constitute the repressive arm. REV-ERB inhibitor and RORA activator form a secondary feedback loop that consolidates the primary loop. It also shows which phases occur in the day (sun) and night (moon). B) Circadian oscillations of different cell types present in the blood and bone marrow. The graphs are based on data taken from Haus et al. (Citation1983), Oh et al. (Citation2019) and Paatela et al. (Citation2019). CCG: Clock controlled gene, WBC: White blood cell, HSPC: Hematopoietic stem and progenitor cells, CT: Circadian time.
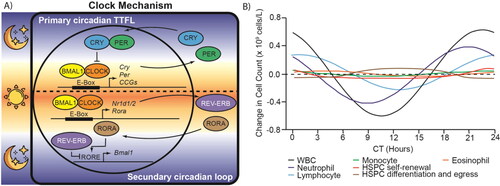
Table 1. Expression of core clock genes in patients with different types of leukemia.
Table 2. Changes in the expression of circadian clock genes after exposure to chemical environmental pollutants.
Figure 3. Impact of aryl hydrocarbon receptor (AhR) activation on circadian rhythms and hematopoiesis. A) The inactive AhR is localized in the cytosol in complex with 90 KDa heat shock protein (HSP90), AhR-interacting protein (AIP), 23 KDa prostaglandin (p23) and cellular-SRC (c-SRC). This can be activated by both endogenous and exogenous (xenobiotic) ligands or agonists. Upon interaction with an agonist, the conformational changes result in the translocation of the complex to the nucleus and the interaction of AhR with BMAL1 after dissociation of the cytoplasmic complex. AhR can compete with CLOCK to form heterodimers with BMAL1 and unlike the CLOCK/BMAL1 dimer that acts as an activator in the promoter E boxes, the AhR/BMAL1 dimer acts as a repressor. B) This causes proteins such as PER1, PER2, CRY1 and CRY2 to not be expressed and, therefore, the amplitude of the circadian oscillations decreases. C) Deregulated AhR signaling in hematopoietic stem and progenitor cells (HSPCs) can cause hematological diseases through a mechanism that is not yet fully understood. AhR overactivation alters bone marrow microenvironment signaling and disrupts quiescence in hematopoietic stem cells (HSCs). Cell proliferation is promoted, and differentiation is altered, favoring mainly the myeloid lineage at the expense of cells of the lymphoid lineage, although AhR overactivation has also been reported in some lymphoid leukemias. LT-HSC: Long-term hematopoietic stem cell, ST-HSC: Short-term hematopoietic stem cell, CMP: Common myeloid progenitor, CLP: Common lymphoid progenitor cell.
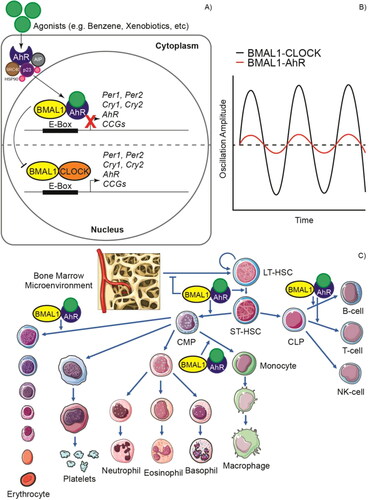
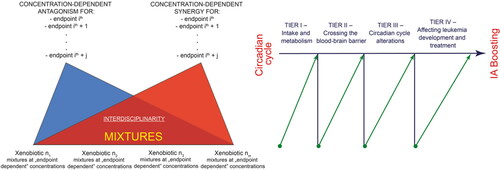
Figure 4. Schematic presentation of possible future experimental developments. We consider four levels: determining pollutants and drug intake, their metabolism/transformation, their arrival at the target organs and tissues, and the eventual development/treatment of leukemia in relation to alterations in circadian cycles.