Figures & data
Figure 1. Total reported numbers of patients infected from a confirmed origin (community or hospital) by specific pathogens under surveillance that show resistance to at least one antibiotic in 2018 (66 countries) and 2019 (70 countries). The data are sourced from the 2020 and 2021 reports of the Global Antimicrobial Resistance and Use Surveillance System (GLASS) that summarize the 2018 and 2019 data, respectively (https://www.who.int/initiatives/glass). The sites of infection and pathogens included in the GLASS are the bloodstream (Acinetobacter spp., Escherichia coli, Klebsiella pneumoniae, Salmonella spp., Staphylococcus aureus, and Streptococcus pneumoniae), urinary tract (E. coli and K. pneumoniae), gastrointestinal tract (Salmonella spp. and Shigella spp.), and genitals (Neisseria gonorrhoeae). Please note that infection cases of unknown origin have been excluded from this analysis and that this figure does not imply a temporal trend due to the evolving number of countries that report to the GLASS.
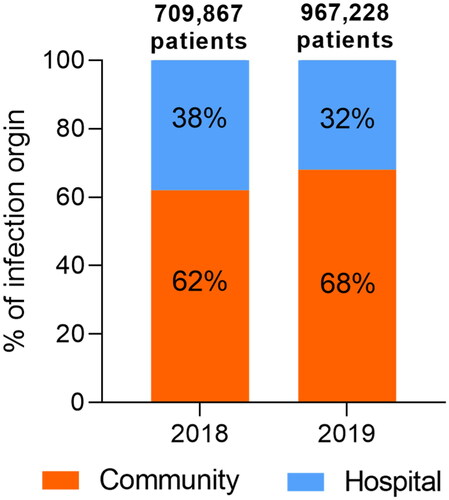
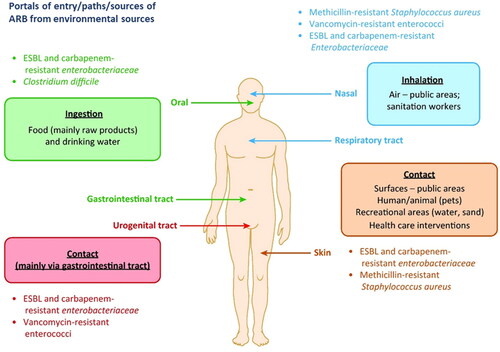
Figure 2. Transmission routes of antibiotic-resistant bacteria (ARB) into humans from environmental sources (Reprinted from Trends in Microbiology, Volume 25, Manaia C.M., Assessing the risk of antibiotic resistance transmission from the environment to humans: Non-direct proportionality between abundance and risk, pp. 173–181, Copyright (2017), with permission from Elsevier).