Figures & data
Figure 1. The hydrogen peroxide-removal system. There are at least three principal nodes for the removal of H2O2. Glutathione peroxidase (GPx) is a selenoenzyme that reduces H2O2 to 2H2O gathering the needed reducing equivalents from glutathione (GSH). The peroxiredoxin (Prx) family of enzymes is a separate node, removing H2O2 using reducing equivalents principally from thioredoxin (Trx). Catalase (CAT) is primarily located in peroxisomes; it requires no reducing cofactors to catalyse the disproportionation of H2O2.
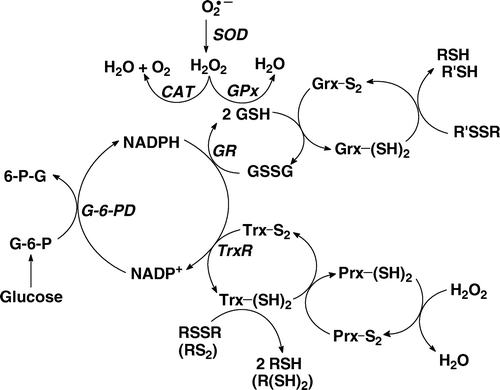
Table I. Rate constants for modeling the kinetic behaviour of GPx Citation[30].
Figure 2. The rate of tumour growth in vivo varies directly with effective GPx activity (i.e. GPx×GSH); H2O2 varies inversely. (♦) Tumour volume, which is proportional to the growth rate. (□) Relative levels of intracellular H2O2 were estimated by monitoring the increase in fluorescence of 2′,7′-dichlorodihydrofluorescein (DCFH2). Effective GPx activity is ‘GPx-activity’ (or GPx) as measured by standard activity assay Citation[44] multiplied by the concentration of GSH. The units are somewhat arbitrary (AU); using typical expressions of the activity of GPx (mU/mg protein) and for GSH levels (nmol/mg protein) units for effective GPx activity would be mU?nmol (mg protein)−2. Figure adapted from Citation[55].
![Figure 2. The rate of tumour growth in vivo varies directly with effective GPx activity (i.e. GPx×GSH); H2O2 varies inversely. (♦) Tumour volume, which is proportional to the growth rate. (□) Relative levels of intracellular H2O2 were estimated by monitoring the increase in fluorescence of 2′,7′-dichlorodihydrofluorescein (DCFH2). Effective GPx activity is ‘GPx-activity’ (or GPx) as measured by standard activity assay Citation[44] multiplied by the concentration of GSH. The units are somewhat arbitrary (AU); using typical expressions of the activity of GPx (mU/mg protein) and for GSH levels (nmol/mg protein) units for effective GPx activity would be mU?nmol (mg protein)−2. Figure adapted from Citation[55].](/cms/asset/f5bc0172-15fb-413a-aaa3-b7bfc1c28090/ifra_a_262357_f0002_b.gif)
Table II. Initial concentrations used for the GPx model.
Figure 3. Model results in determining linear dependency of time constant with respect to effective GPx activity. The dependence of the time constant, τ, on effective GPx activity, approximated by [GPxr]0[GSH]0, are shown on log-log plots for various initial concentrations of GPxr and H2O2. Results from the general model are shown as solid lines; results from the classical model are shown as dotted lines. The short segments result from calculations of effective GPx activity for a fixed [GPxr]0 with a span on [GSH]0 (0.1, 0.2, 0.4 and 0.6 mM). If the line segments are parallel to the abscissa, then there is no dependence of τ on [GSH] in the range of concentrations tested; if the line-segments show a non-zero slope, then there is dependence of [GSH]. The system would be completely dependent on effective GPx activity if all points fell on a single straight line. [GPxr]0 used for both models are 0.1 µm, 0.5 µm, 1 µm, 5 µm, 10 µm and 50 µm. (a) [H2O2]0 = 0.1 µm; (b) [H2O2]0=0.5 µm; (c) [H2O2]0=1 µm; (d) [H2O2]0 = 5 µm; (e) [H2O2]0=10 µm; (f) [H2O2]0=50 µm. The general model captures dependency when [H2O2]0 is 5 µm and [GPxr]0 is ≤1 µm. Note that the classical model under-predicts the [H2O2]0 for the onset of effective GPx activity dependency. Furthermore, the estimated time constants for the classical model can be orders of magnitude different than that determined from the more general kinetic model.
![Figure 3. Model results in determining linear dependency of time constant with respect to effective GPx activity. The dependence of the time constant, τ, on effective GPx activity, approximated by [GPxr]0[GSH]0, are shown on log-log plots for various initial concentrations of GPxr and H2O2. Results from the general model are shown as solid lines; results from the classical model are shown as dotted lines. The short segments result from calculations of effective GPx activity for a fixed [GPxr]0 with a span on [GSH]0 (0.1, 0.2, 0.4 and 0.6 mM). If the line segments are parallel to the abscissa, then there is no dependence of τ on [GSH] in the range of concentrations tested; if the line-segments show a non-zero slope, then there is dependence of [GSH]. The system would be completely dependent on effective GPx activity if all points fell on a single straight line. [GPxr]0 used for both models are 0.1 µm, 0.5 µm, 1 µm, 5 µm, 10 µm and 50 µm. (a) [H2O2]0 = 0.1 µm; (b) [H2O2]0=0.5 µm; (c) [H2O2]0=1 µm; (d) [H2O2]0 = 5 µm; (e) [H2O2]0=10 µm; (f) [H2O2]0=50 µm. The general model captures dependency when [H2O2]0 is 5 µm and [GPxr]0 is ≤1 µm. Note that the classical model under-predicts the [H2O2]0 for the onset of effective GPx activity dependency. Furthermore, the estimated time constants for the classical model can be orders of magnitude different than that determined from the more general kinetic model.](/cms/asset/e4c2ad20-238b-4a61-b5fd-7667dcc910bd/ifra_a_262357_f0003_b.jpg)
Figure 4. Example cases demonstrating deviations between the generalized and classical model results. Cases used here, as examples to demonstrate discrepancies, are for initial concentration of H2O2, [H2O2]0, of 5 µm and initial concentration of GSH, [GSH]0, of 0.1 mm. (A) Transient H2O2 profiles for various [GPxr]0 of the general (solid lines) and classical (dotted lines) models are shown in this semi-log plot. Even for cases with lower [GPxr]0, where the time needed for 63% decay (time constants, τ) from both models agree well, the classical model is not a good approximation throughout. For example, in the case where [GPxr]0 is 1 µm (magenta), the time taken for 10% decay given by both models are a factor of 10 different. (b) The rates of disappearance of H2O2 for various [GPxr]0 of the general (solid lines) and classical (dotted lines) models are plotted with a semi-log scale. Using the same case of where [GPxr]0 is 1 µm (magenta), at physiological turnover time for H2O2 of ms, the rate of removal of H2O2 given by the general model is approximately a factor of 100 greater than the classical rate.
![Figure 4. Example cases demonstrating deviations between the generalized and classical model results. Cases used here, as examples to demonstrate discrepancies, are for initial concentration of H2O2, [H2O2]0, of 5 µm and initial concentration of GSH, [GSH]0, of 0.1 mm. (A) Transient H2O2 profiles for various [GPxr]0 of the general (solid lines) and classical (dotted lines) models are shown in this semi-log plot. Even for cases with lower [GPxr]0, where the time needed for 63% decay (time constants, τ) from both models agree well, the classical model is not a good approximation throughout. For example, in the case where [GPxr]0 is 1 µm (magenta), the time taken for 10% decay given by both models are a factor of 10 different. (b) The rates of disappearance of H2O2 for various [GPxr]0 of the general (solid lines) and classical (dotted lines) models are plotted with a semi-log scale. Using the same case of where [GPxr]0 is 1 µm (magenta), at physiological turnover time for H2O2 of ms, the rate of removal of H2O2 given by the general model is approximately a factor of 100 greater than the classical rate.](/cms/asset/3aa6b4fc-6f2b-4d3b-ae1e-e72806bbe477/ifra_a_262357_f0004_b.gif)
Figure 5. Example of the rate-limiting effect of the slower GPxr recycling step. Concentration profiles of H2O2 and GPxr of the general model are shown for [H2O2]0 of 5 µm, [GSH]0 of 0.1 mM and [GPxr]0 of 1 µm. The inflection point on the [H2O2]-profile that occurs around 40 ms corresponds to the change in the rate of production of GPxr.
![Figure 5. Example of the rate-limiting effect of the slower GPxr recycling step. Concentration profiles of H2O2 and GPxr of the general model are shown for [H2O2]0 of 5 µm, [GSH]0 of 0.1 mM and [GPxr]0 of 1 µm. The inflection point on the [H2O2]-profile that occurs around 40 ms corresponds to the change in the rate of production of GPxr.](/cms/asset/619f458a-341a-4630-9873-ef9b27dda97a/ifra_a_262357_f0005_b.gif)