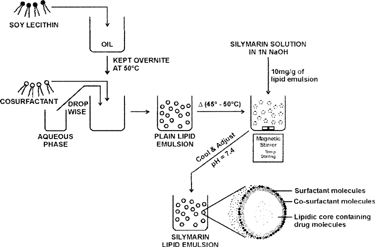
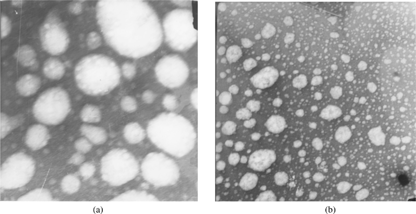
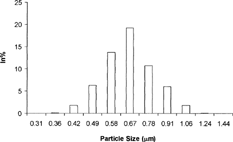
Figures & data
Effect of various oils on the plain lipid emulsion formation and stability characteristics at room temperature (20 ± 5°C)
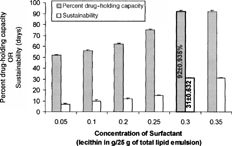
2 Effect of varying concentrations of surfactant (lecithin) on percent drug-holding capacity and sustainability of silymarin lipid emulsion.
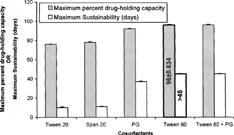
3 Effect of different cosurfactants on maximum percent drug-holding capacity and maximum sustainability of silymarin lipid emulsion.
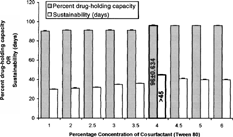
4 Effect of varying concentrations of cosurfactant (Tween 80) on percent drug-holding capacity and sustainability of silymarin lipid emulsion.
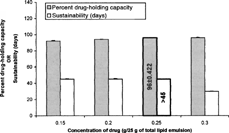
5 Effect of different levels of drug on the percent drug-holding capacity and sustainability in final composition of the lipid emulsion.
Oil phase separation studies of formulation A at room temperature (20 ± 5°C), at higher temperature (40 ± 5°C), and at lower temperature (4°C)
Effect of centrifugation (4000 rpm) on oil phase separation
Effect of different pH on formulation A (at 20 ± 5°C)
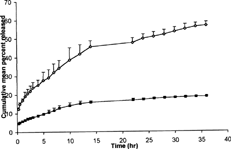
Drug leakage studies of formulation A at room temperature (20 ± 5°C) during storage period
Composition of silymarin formulations