Figures & data
TABLE 1 Blood glucose levels and body weight change following once a 2 week-treatment (200 U/kg/2 wk) of the insulin in formulation
TABLE 2 Blood glucose levels and body weight change following once a 2 week-treatment (125 U/kg/2 wk) of the insulin formulation
TABLE 3 Blood glucose levels and body weight change following once a 2 week-treatment (125 U/kg/2 wk) of the insulin formulation
FIG. 2 Blood glucose level and body weight following every 10 days-treatment (125 U/kg) of insulin-containing PLGA microcapsules to BB/wor//Tky diabetic rats BB/Wor//Tky hyperglycemic pregnant rats were subcutaneously given 125 U/kg of PLGA microcapsules containing 3 w/w% insulin every 2 weeks. Blood glucose level (upper) and body weight (lower) were monitored. Mean ± SD. Female n=6; male n=8. *p < 0.05 vs. the first dosing day; #p < 0.05 vs. male group; body weight in male group was significantly higher throughout the experiment.
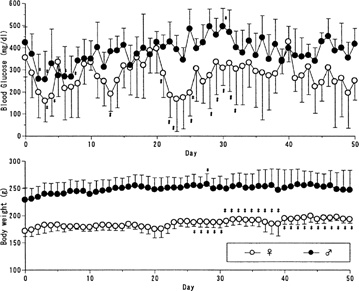
TABLE 4 The trial of intermittent treatment (125 U/kg sc every 10 days) to BB/Wor//TKy pregnant rats
FIG. 3 Trial of intermittent administration of the formulation to BB pregnant rats BB/Wor//Tky hyperglycemic pregnant rats were subcutaneously given 125 U/kg of the insulin formulation every 10 days. Blood glucose level (○) and body weight change (▴) were monitored. Arrow represents the birth of fetuses.
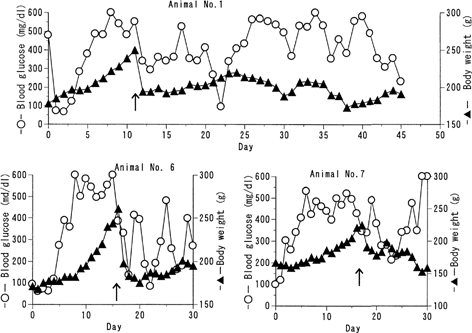
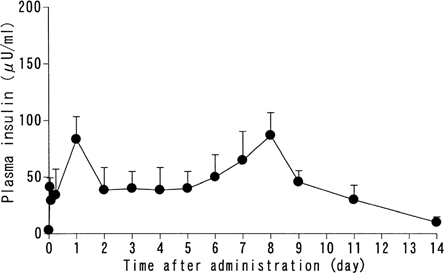
FIG. 1 Plasma insulin level following a single subcutaneous administration of insulin-containing PLGA microcapsules PLGA microcapsules containing 3 w/w% insulin were subcutaneously administered (125 U/kg) as a single dose to STZ-induced hyperglycemic rats. Plasma insulin levels were monitored. Mean ± SD, n = 5.