Figures & data
Figure 1 The molecular structure of D-alanine N-mustard is shown with the N-mustard group indicated by inset rectangle and sites of alkylation to nucleophiles by inset arrows (SMILES nomenclature is shown). C-13 assignments for numbered carbon atoms are as follows (number/ppm): 2/172.0; 4/69.5; 5/44.6; 7/59.0; 9/53.1; 10/44.7; 12/14.9; 13/53.1; 14/44.7. Chlorine atoms 15, 11, and 6 are removed upon alkylation of a nucleophile.
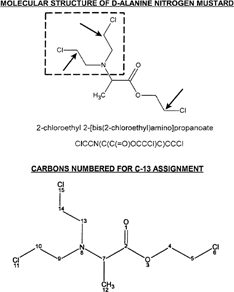
Figure 2 General synthesis represented here shows a two-step approach, where the formation of an ammonium carboxylate is the required first step. Triethylamine serves as a proton sink to protect the ester group that is formed after the second step. A mild reflux in the presence of 1,2-dichloroethane will result in the product having the chloroethyl (ClCH2CH2-) substituents.
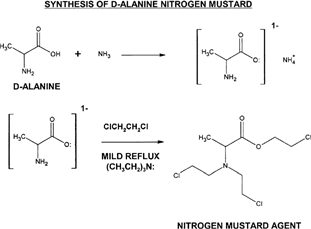
Figure 3 Alkylation of p-chloroaniline is shown here with zero-order plot of reaction kinetics. Note that the N-mustard agent has a total of three potential sites for reaction with nucleophiles, with two remaining (see inset arrows) after the first alkylation reaction. Absorbance versus time data falls within a 95% confidence interval (see lower portion) giving a zero-order rate constant (k=7.445E-04 mol/l/min) from the slope of the regression line.
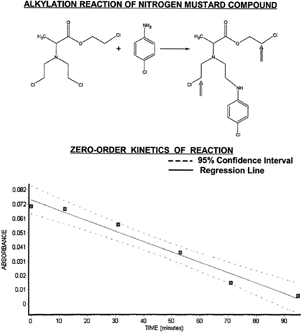
Table 1 Molecular properties of D-alanine nitrogen mustard
Table 2 Molecular properties of D-alanine nitrogen (N) mustard and clinical agents
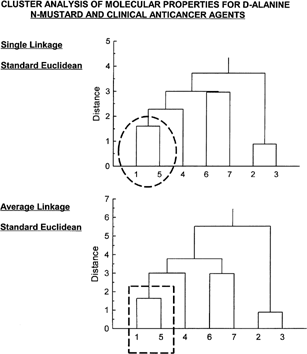
Figure 4 Cluster analysis of properties shown in for the seven compounds. Both single linkage and average linkage analysis (standard Euclidean for both) show D-alanine N-mustard (1) to be highly similar to cyclophosphamide (5). Drug 6 (chlorambucil) is most similar to drug 7 (melphalan). Drug 2 (mechlorethamine) is likewise most similar to drug 3 (mustargen).