Figures & data
FIG. 1 Mean levonorgestrel plasma concentrations versus time profiles for oral and nasal administration (mean ± SD, n = 4). ♦-LNG suspension by oral administration at 500 μg/rat; ▴-EPC/SG/LNG by nasal administration at 500 μg/rat; ▵-EPC/SG/LNG/chitosan by nasal administration at 500 μg/rat; •-EPC/SG/LNG by nasal administration at 350 μg/rat; ▪-EPC/Ch/LNG by nasal administration at 350 μg/rat.
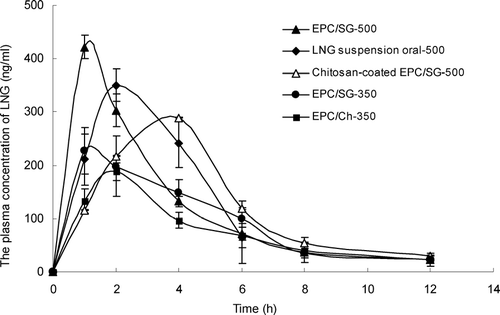
TABLE 1 Pharmacokinetic parameters of different levonorgestrel (LNG) formulations after oral or nasal administration in rats*
TABLE 2 Contraceptive effects with different levonorgestrel (LNG) formulations