Figures & data
FIG. 1 Viability of bifidobacteria in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers. * Significantly different from unprotected bifidobacteria cultures.
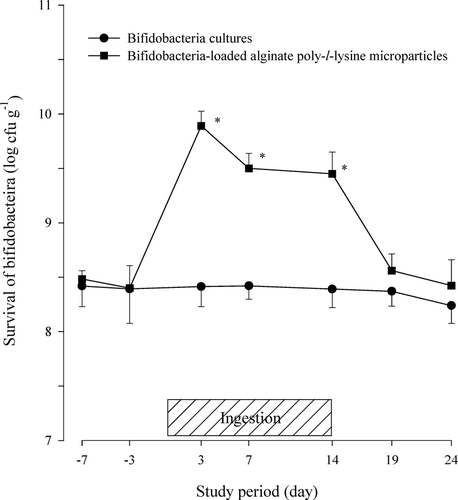
FIG. 2 Viability of lactic acid bacteria in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers.
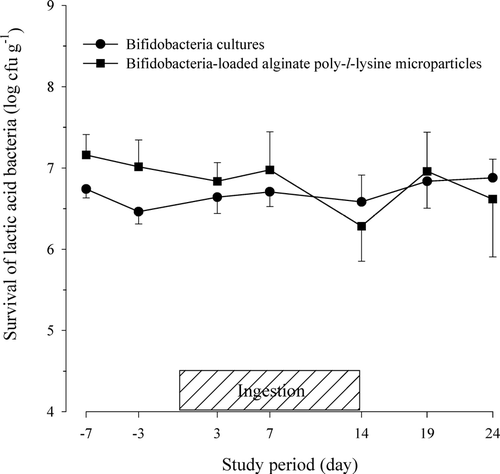
FIG. 3 Viability of enterobacteriaceae in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers.
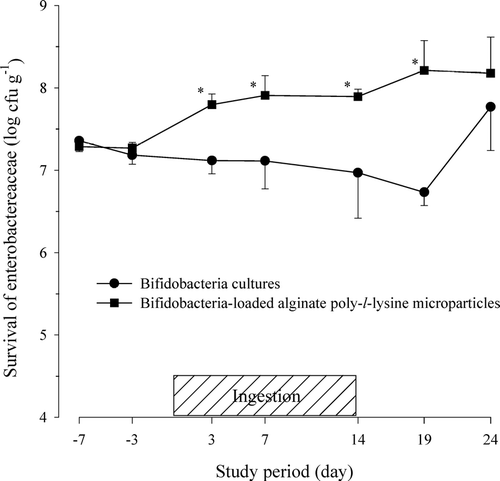
FIG. 4 Viability of staphylococci in feces after the oral administration of protected bifidobacteria-loaded alginate poly-l-lysine microparticles in healthy human volunteers.
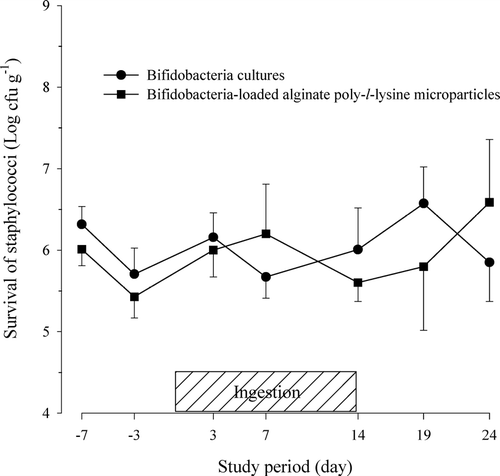
TABLE 1 Intervals between bowel movements (days) after the oral administration of unprotected bifidobacteria cultures (group A) and protected bifidobacteria-loaded alginate poly-l-lysine microparticles (group B) to healthy human volunteers
TABLE 2 Number of volunteers by scoring indices of fecal volume after the oral administration of unprotected bifidobacteria cultures (group A) and protected bifidobacteria-loaded alginate poly-l-lysine microparticles (group B)
TABLE 3 Number of volunteers by scoring indices of fecal viscosity after the oral administration of unprotected bifidobacteria cultures (group A) and protected bifidobacteria-loaded alginate poly-l-lysine microparticles (group B)