Figures & data
FIG. 1 Nanoparticle size distribution. (A) and (B) Size distribution of nanoparticles (PLGA-α -TEA) determined by dynamic light scattering, using a neon laser of λ = 677.0 nm and measured at 90° degree. The effective diameter of nanoparticles = 272 nm, polydispersity = 0.313. Measurements were repeated three times, each time with similar patterns. (C) Transmission electron microscopy of α -TEA-loaded nanoparticles (PLGA: α -TEA = 10:1). Nanoparticles were visualized directly after freeze-drying. The mean diameter was 190 ± 65 nm. Bar represents 500 nm.
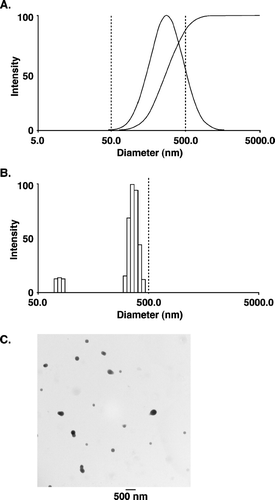
FIG. 2 Effects of liposome- or nanoparticle-formulated α -TEA delivered by gavage on tumor burden. 66cl-4-GFP cells at 2 × 105/mouse were injected into the inguinal area at a point equal distance between the fourth and fifth nipples. Then 12 days after tumor injection, mice were assigned to control and α -TEA treatment groups such that the mean tumor volume of each group was closely matched (average tumor volume/group = 1.88 mm3). Mice (10/group) were treated with liposomal control, nanoparticle control, liposomal α -TEA, or nanoparticle α -TEA at high dose (5 mg/mouse/day) or low dose (2.5 mg/mouse/day) for 20 days. Tumor volumes (mm3) are depicted as mean ± SE. (1) = significantly reduced in comparison to liposome control, (2) = significantly reduced in comparison to nanoparticle control, and (3) = significantly reduced in comparison to liposomal α -TEA at 2.5 mg/day.
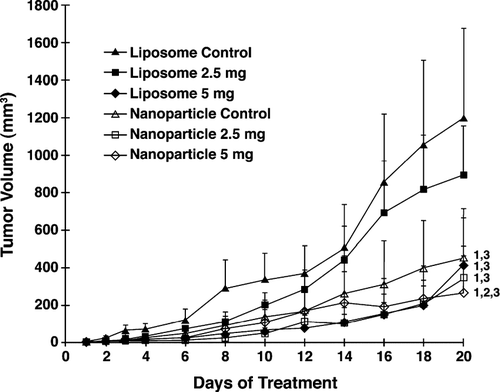
TABLE 1 α -TEA formulated into liposome or nanoparticle reduced the average number of visible macroscopic lung metastases.
FIG. 3 Effects of liposome- and nanoparticle-formulated α -TEA delivered by gavage on micrometastatic tumor foci in lung and lymph node. (A) Using a Nikon fluorescence microscope, the number of fluorescent microscopic tumor foci on the surface (top and bottom) of flattened left lung lobes was determined for control and treatment groups. Micrometastatic tumor foci were grouped into four size groupings (< 20 μ m, 20-50 μ m, 50–100 μ m, or > 100 μ m). (B) Using a Nikon fluorescence microscope, the number of fluorescent microscopic metastases in brachial and axillary lymph nodes was determined for control and treatment groups. For both (A) and (B), average number of total lesions was calculated. Data are depicted as mean ± SE of total number of microscopic metastases. (1) = significantly reduced in comparison to liposome control, (2) = significantly reduced in comparison to nanoparticle control, and (3) = significantly reduced in comparison to liposomal α -TEA at 2.5 mg/day.
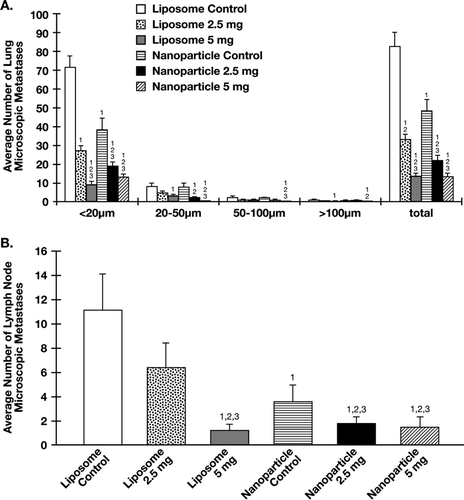
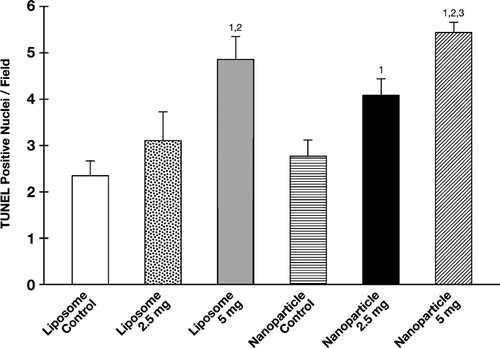
FIG. 4 α -TEA formulated into liposome or nanoparticle and delivered by gavage-induced 66cl-4-GFP cells to undergo apoptosis. Fully 5 μ micron tumor tissue sections from control and treatment groups were examined for levels of apoptosis by TUNEL. Data are depicted as mean ± SE of TUNEL positive-stained cells/field. (1) = significantly enhanced in comparison to liposome control, (2) = significantly enhanced in comparison to nanoparticle control, and (3) = significantly enhanced in comparison to liposomal α -TEA at 2.5 mg/day.