Figures & data
Table 1. The characteristics of the selected formulations.
Table 2. The composition, dose and route of administration of the selected formulations.
Figure 1. Mean plasma AMB concentration–time profiles after intravenous administration of 1.0 mg/kg of F1-iv and oral administrations of 10 mg/kg of F1-PO and Fungizone® to rats (n = 6).
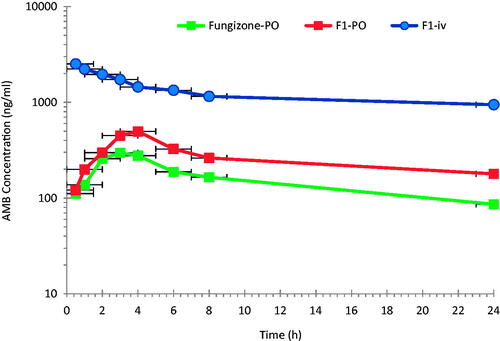
Table 3. Pharmacokinetic parameters of AMB after iv and oral administrations of AMB-loaded PLGA-PEG formulations and fungizone in rats (n = 6).
Figure 2. Mean plasma AMB concentration–time profiles following single oral administration of 10.0 mg/kg of AMB-loaded PLGA-PEG formulations in rats (n = 6).
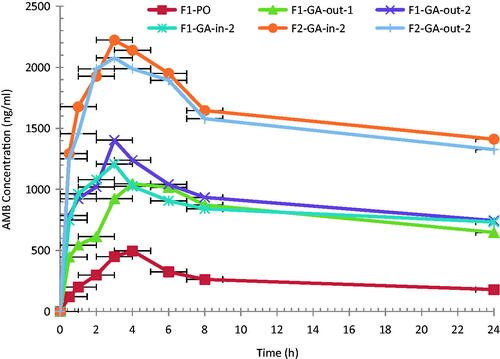
Table 4. Pharmacokinetic parameters of AMB (mean ± SD) after a 10 mg/kg single oral administration of AMB as Fungizone® and AMB-loaded PLGA-PEG NP formulations in rats (n = 6).
Figure 3. BUN, mg/dL, (mean ± SD) after single and multiple iv doses (5 mg/kg) of AMB and AMB loaded to PLGA-PEG NP to rats (n = 3).
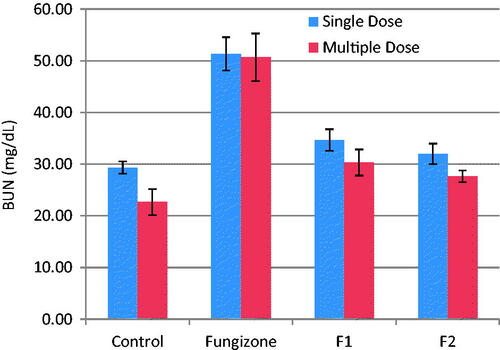
Figure 4. Plasma Cr, mg/dL, (mean ± SD) after single and multiple iv doses (5 mg/kg) of AMB and AMB loaded to PLGA-PEG NP to rats (n = 3).
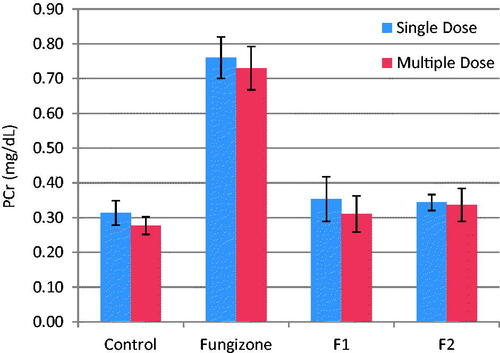
Figure 5. Typical kidney tissue alterations verified in rats treated with AMB or its equivalent dose as 1.0 mg/kg of body weight as iv administration of different Amb-PLGAPEG copolymer. 1) normal kidney tissue; 2,3,4) Fungizone® f1 and f2, respectively, varying degree of nephrotoxicity necrosis related to iv administration of iv doses (5 mg/kg).
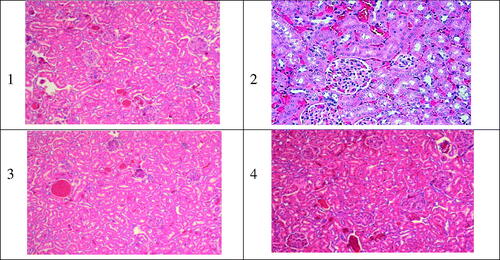
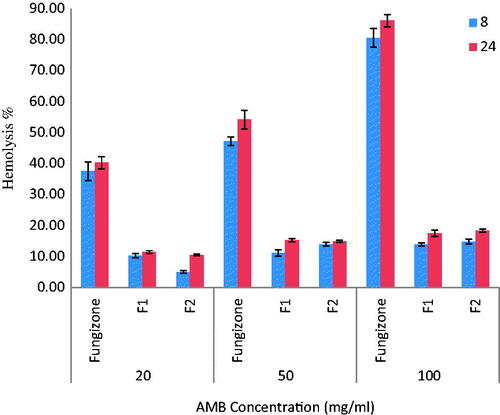
Figure 6. In vitro mean RBCs hemolysis following incubation of RBC with Fungizone® and different AMB-NPs formulations at concentrations of 20, 50 and 100 μg/ml (n = 3).