Figures & data
Table 1. In-vitro evaluation of curcumin in-situ gel formulations containing 30% pluronic F127 and 1% carbopol P934, of pH 4 at zero time and after three months storage in refrigerator.
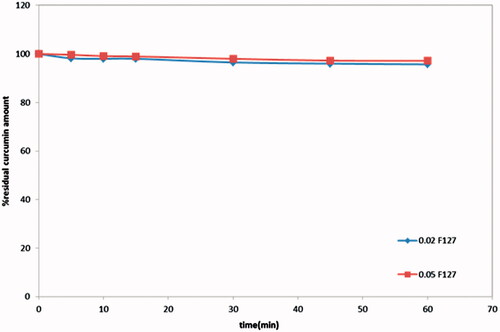
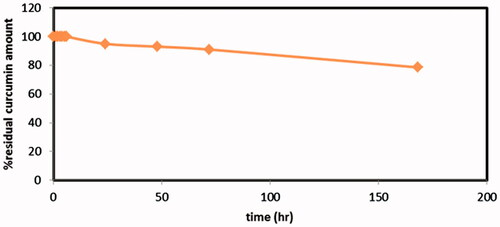
Figure 4. In-vitro release study of curcumin in-situ gel formulations: (a) without neutralization; (b) with neutralization.
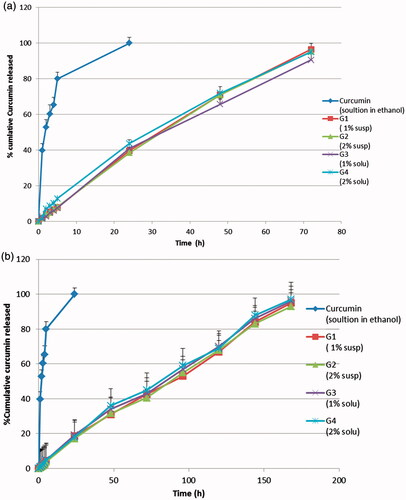
Table 2. Release Models for the selected formulation with and without neutralization.
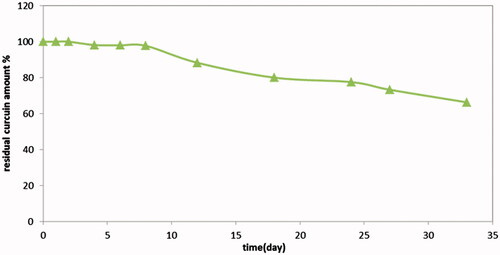
Figure 5: Drug release profiles of Cur in-situ gel formulations subjected to a stability study in refrigerator, for 3 months. (a) G1 containing 1% suspended Cur formulation, (b) G2 containing 2% suspended Cur formulation, (c) G3 containing 1% dissolved Cur formulation and (d) G4 containing 2% dissolved Cur formulation.

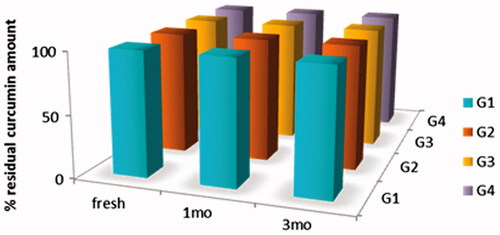
Figure 6. Drug content of Cur in-situ gel formulations subjected to a stability study in refrigerator, for 3 months.