Figures & data
Table 1. Composition and pre-compression characterization of risperidone liquisolid formulae.
Table 2. Post-compression characterization of risperidone liquisolid formulation tablets.
Figure 2. Dissolution data of risperidone from different prepared liquisolid tablet formulae in comparison to plain drug and conventional tablets.
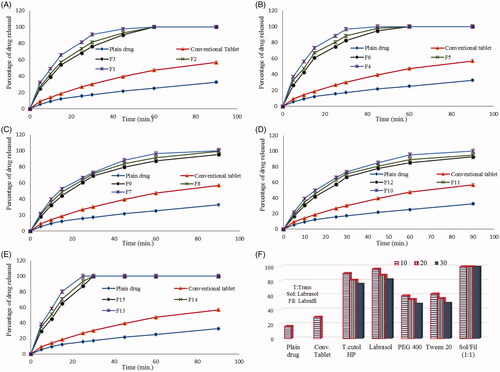
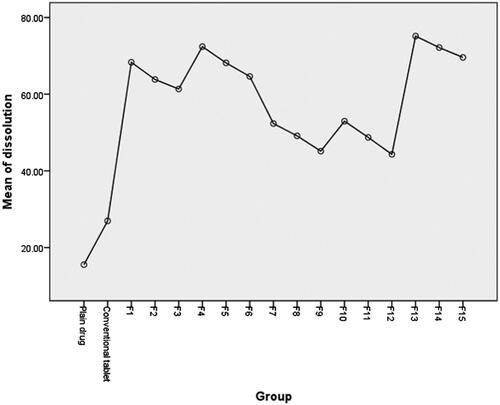
Table 3. One way ANOVA test of dissolution data.
Figure 4. Solid state characterization of risperidone liquisolid formula (F13) using A) DSC, B) IR and C) XRD.
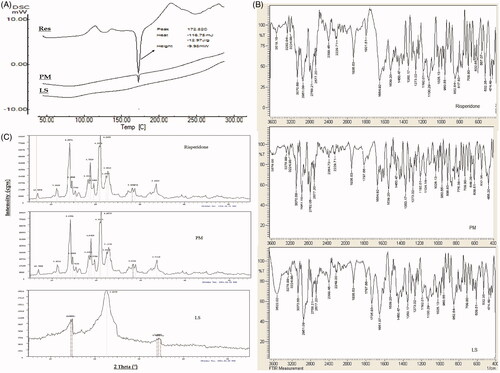
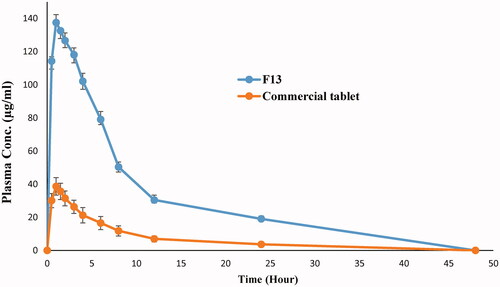
Figure 5. Mean plasma concentration time curve of risperidone liquisolid formula (F13) and conventional tablet in rabbits.