Figures & data
Figure 1. Schematic of the injectable and in situ-forming implants for tumor therapy. This injectable implant presents a liquid–solid phase transformation that is responsive to body fluid or blood. Chemotherapy drugs and ethyl oleate can be prepared in advance and stored in the refrigerator for a long time. Before use, the drug and ethyl oleate suspension are mixed with the NBCA and then injected into the animal body. NBCA will polymerize immediately at the injection site, thus forming a drug depot.
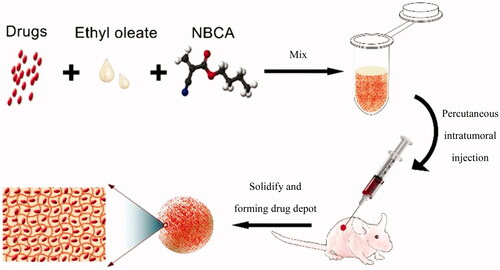
Figure 2. (A) TEM image of the paclitaxel-ethyl oleate suspension. The particles of paclitaxel in the ethyl oleate suspension are rice shaped, the length is approximately 0.5–1 μm, and the diameter is 0.5 μm. (B and C) Drug-loaded NBCA-ethyl oleate implants prepared in vitro. (B) Paclitaxel-loaded 30% NBCA of INEI; (C) epirubicin-loaded 30% NBCA of INEI. When water or deionized water was not added, the NBCA implants did not solidify for several days. Once a small amount of saline was added, the implants quickly solidified. (D–G) FESEM images of the NBCA-ethyl oleate implants. (B) 30% NBCA, (C) 50% NBCA, (D) 80% NBCA, and (E) 100% NBCA. NBCA is triggered by the anion and then self-crosslinks to form particles, which then cross-link with each other to form a solid material. The larger the particles, the greater the gaps formed between the particles.
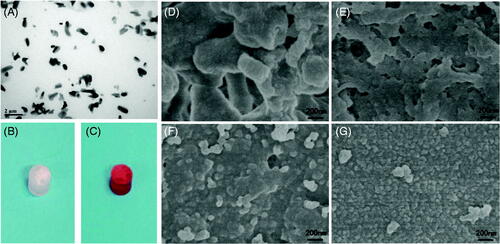
Figure 3. Time course of the drug release ratio in vitro. (A) Release ratio of paclitaxel and paclitaxel loaded in an INEI with 30% NBCA; and (B) release ratio of epirubicin and epirubicin loaded in an INEI with 50% NBCA. The concentration of released paclitaxel and epirubicin in the sodium salicylate solution was quantified as described in the Materials and methods section. The amount of initially incorporated molecules in the NBCA implants is defined as 100%. Each data point represents the mean ± SD of triplicate measurements. (C) Biodegradability of the NBCA implant in vivo. The implants were subcutaneously injected into the mice, removed after 1 h and weighed, and this weight was defined as 100%. One mouse from each group was sacrificed at 1 week intervals, and then the implants were removed and weighed. Each data point represents the mean ± SD of triplicate measurements (n = 3).
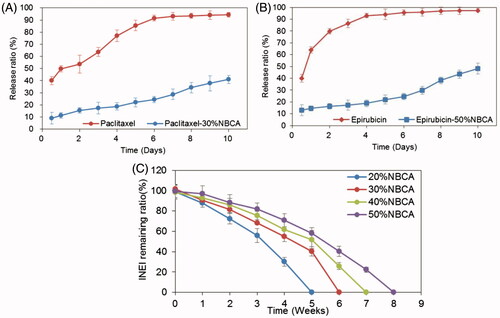
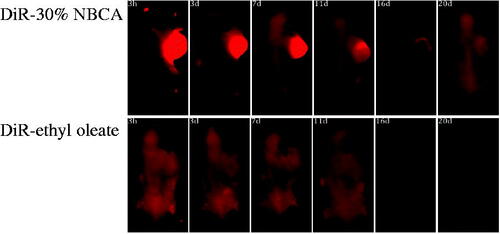
Figure 4. Effect of the NBCA implant on the retention of fluorescent dye DiR in the tumor: DiR-30% NBCA (top) and DiR-ethyl oleate solution (bottom).