Figures & data
Figure 1. Constrained simplex-centroid design for optimization of a ternary surfactant mixture (upper panel), and ternary response surface plots showing the effect of mixture composition on measured responses (lower panel).
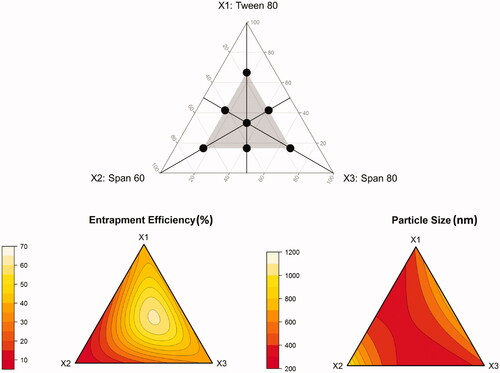
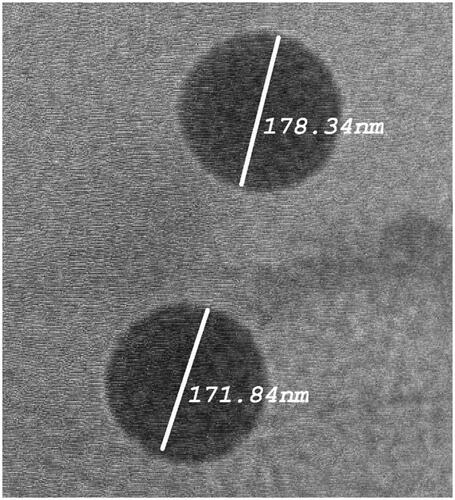
Table 1. ANOVA of responses measured according to the Plackett–Burman design.
Figure 3. LTD in vitro release (A) and permeation across chicken buccal mucosa (B) from the transferosomal gel relative to the control gel. Plot (C) is correlation of percentage LTD permeated and released from the transferosomal and control gels.
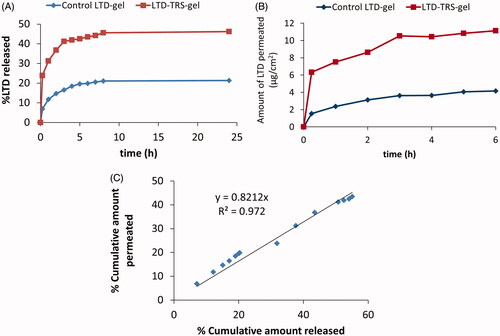
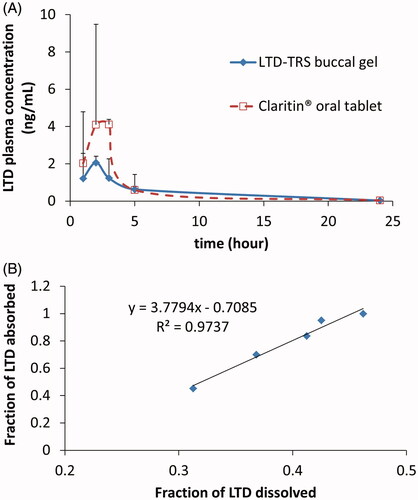
Figure 4. Average (+SD) Plasma LTD cementations following administration of 10 mg LTD in transferosomal buccal gel and marketed Claritin® tablet in three healthy human volunteers (A), and Level A IVIVC plot for LTD in the transferosomal buccal gel (B). Plasma concentrations of both formulations at each time point in plot (A) were not significantly different on ANOVA test (p > 0.05).