Figures & data
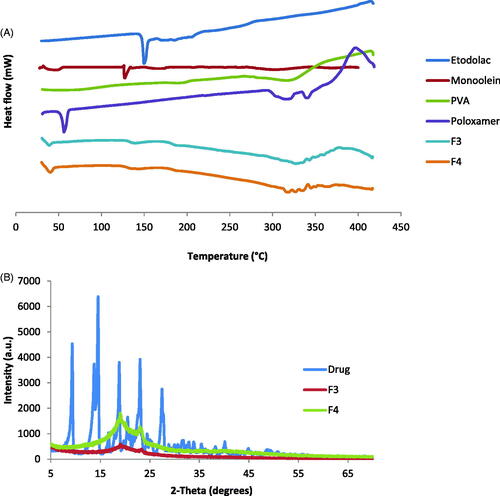
Table 1. Composition and characterization parameters values for etodolac loaded cubosomes nanoparticles.
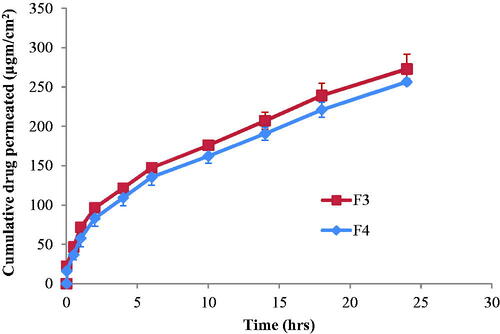
Table 2. Release parameter values for etodolac-loaded cubosomes nanoparticles.
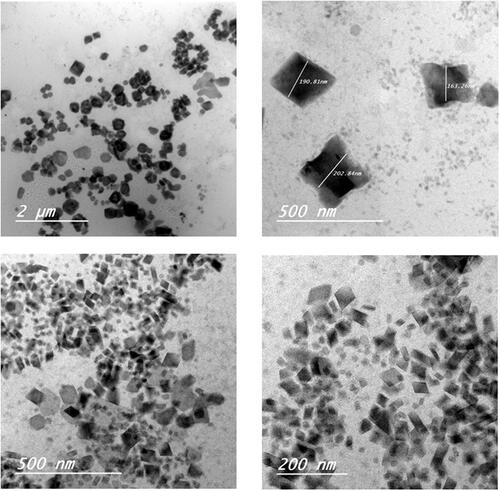
Figure 1. Transmission electron microscopy (TEM) images of etodolac cubosomes nanoparticles: a and b for F3 and c and d for F4.