Figures & data
Table 1. Composition of prepared MSP-PLCPs, percent yield, n-octanol/water partition coefficient (P), water solubility, n-octanol solubility, in vitro mucoadhesion time particles size, and PDI of MSP and MSP-PIPs.
Table 2. Composition, disintegration time, wetting time, USP Q3, USP Q10, SSDT Q2, SSDT Q10, mucoadhesion time, and bioadhesion force of the prepared tablets.
Figure 2. TEM micrographs of optimum MSP-PLCP (a and b) and TEM micrographs of the optimized tablet dispersion (c and d) in simulated saliva.
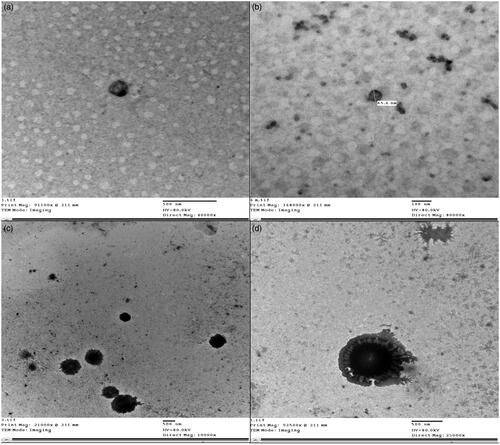
Figure 3. The effect of mucoadhesive polymer concentration on (a) disintegration time, (b) wetting time, (c) SSDT Q2 and (f) bioadhesion force. Combined effect of mucoadhesive polymer concentration and type on (d) SSDT Q10 and (e) mucoadhesion time. Desirability curve for optimization (g).
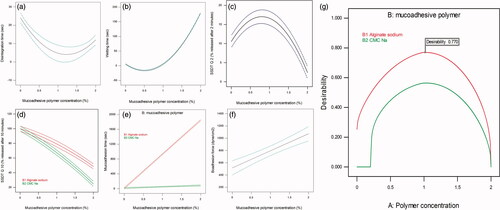
Table 3. Summary of the pharmacokinetic parameters of MSP following the sublingual administration of Fluxopride® and the optimized test formulation.