Figures & data
Table 1. Formulation of CbFG containing permeation enhancer.
Table 2. Inter- and Intra-data on ketoprofen analysis using HPLC.
Figure 1. Transparency changes of CbFG-film during film formation; (A) initial state after CbFG application, (B) 5 min after CbFG application; (C) 10 min after CbFG application.

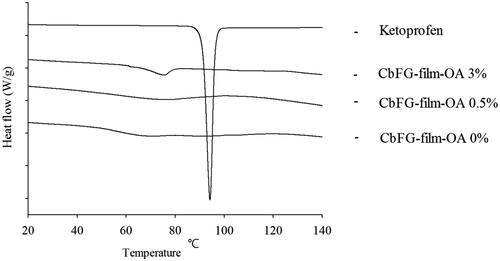
Figure 2. Mechanical properties of CbFG-films; (A) film tensile strength, (B) maximum elongation at break. One-way ANOVA analysis, *p < 0.05 (n = 4) compared with film containing OA 0%.
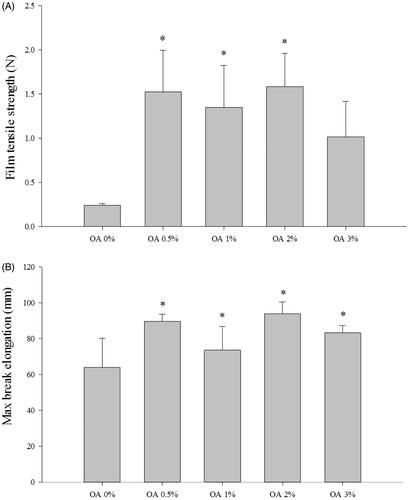
Figure 7. In vitro skin permeation profiles of CbFG-film containing ketoprofen; (A) with different kinds of enhancers through excised SD-rat skin, (B) with different amount oleic acid as an enhancer through excised hairless mouse skin. One-way ANOVA analysis,* p < 0.05 (n = 3) compared with Commercial product.
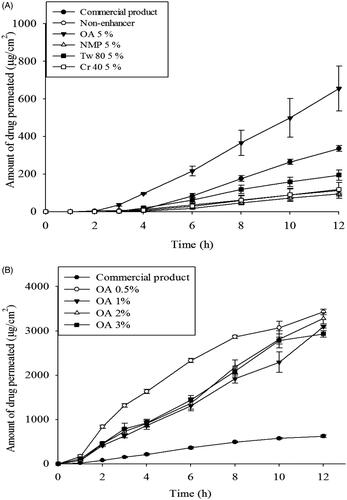
Table 3. In vitro skin permeation rate and lag time of CbFG-film through excised SD-rat skin and hairless mouse skin.
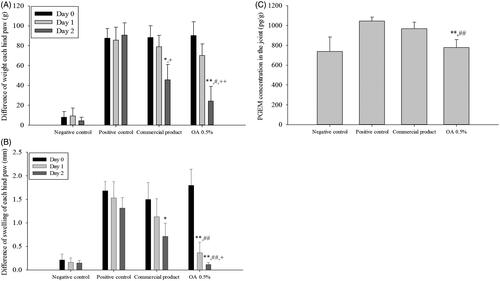
Figure 8. In vivo efficacy study of CbFG-film-OA 0.5% and commercial product; (A) difference in swelling of each hind paw, (B) difference in weight of each hind paw, (C) PGEM levels in joint tissue for 2 days after administration. One-way ANOVA analysis: *p < 0.05 (n = 6), compared with positive control; **p < 0.05 (n = 6), compared with positive control; #p < 0.05 (n = 6), compared with negative control; ##p < 0.05 (n = 6), compared with negative control; +p < 0.05 (n = 6), compared with Commercial product; ++p < 0.01 (n = 6), compared with Commercial product.