Figures & data
Table 1. The sol–gel transition temperature of agomelatine loaded SLNs in situ gels prepared using 18% w/v of Pluronic F 127 and 0.2% w/v of different types of mucoadhesive polymers.
Table 2. Experimental runs, independent variables and measured responses of the full factorial experimental design of agomelatine-loaded SLNs in situ gels.
Figure 1. Release profiles of the full factorial design agomelatine loaded solid lipid nanoparticles in situ gel formulae (A) G1–G6) and (B) G7–G12 in phosphate buffer (pH 6.8) and at 37 °C.
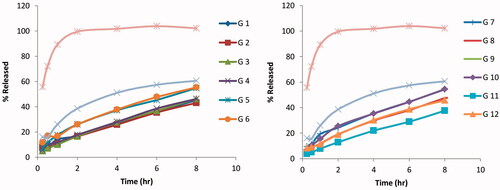
Figure 2. Transmission-electron micrograph (TEM) of the optimized agomelatine loaded solid lipid nanoparticles in situ gel formula.
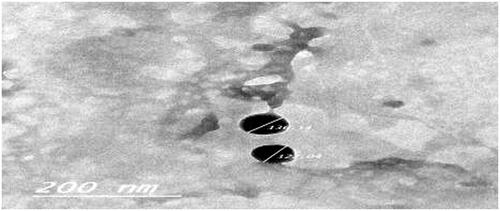
Figure 3. Agomelatine (A) mean plasma concentrations and (B) mean brain concentrations after administration of intranasal Gel-3, IV agomelatine solution, and oral agomelatine suspension.
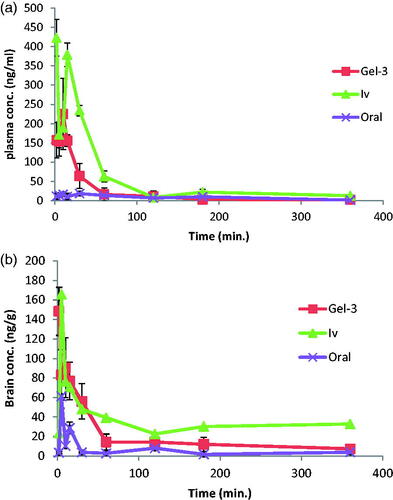
Table 3. Pharmacokinetic parameters of agomelatine in both plasma and brain.
