Figures & data
Figure 1. Schematic illustration of the devices and the results of adhesive strength evaluation (n = 3). (A) the schematic illustration of the devices used for evaluation of adhesive strength, A1 for tensile stress and A2 shear stress; (B) adhesive strength results of the ETGFA gel and the commercial vaginal gel Asimi®.
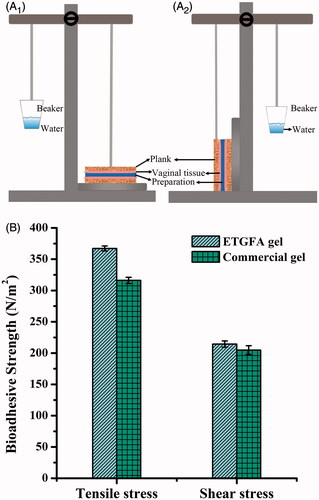
Figure 2. Comparison of expansion height and duration time of foam (n = 3) propelled by (A) hydrocarbon (propane/butane = 80/20, v/v) ranging from 3 to 10%, w/w as well as (B) dimethyl ether, ranging from 6 to 20%, w/w; and the foam appearances from expansion to collapse (25.0 ± 0.5 °C) at 0 min (C1), 2 min (C2), 10 min (C3) and 60 min (C4), respectively.
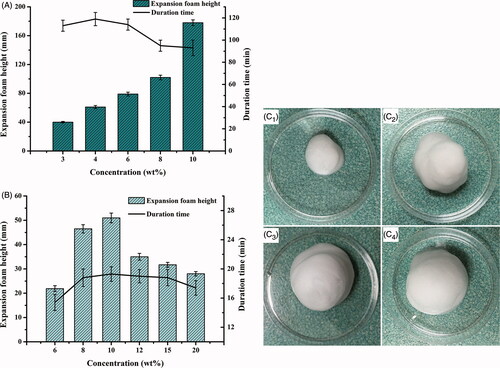
Figure 3. Rheological properties of the ETGFA. (A) viscosity of the ETGFA at 25.0 ± 0.5 and 37.0 ± 0.5 °C, respectively; (B) viscoelasticity of the ETGFA from 25 to 32 °C; (C) viscoelasticity of the ETGFA diluted with stimulus vaginal fluid from 32 to 37 °C and (D) gelation time of the ETGFA diluted with stimulus vaginal fluid from foam to gel at 37.0 ± 0.5 °C.
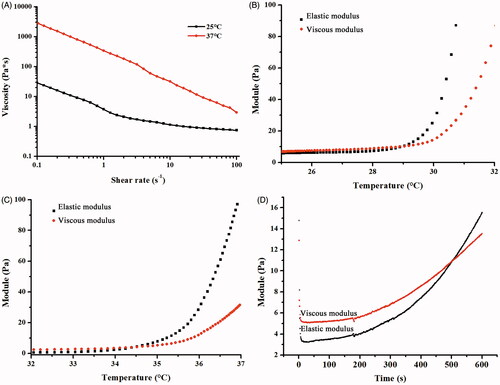
Figure 4. In vitro drug release and antimicrobial effect. (A) in vitro drug release from the ETGFA and the Asimi® determined by membrane-free method at 37.0 ± 0.5 °C (n = 3); (B) MICs of silver nanoparticle solution and the drug-loaded ETGFA on P. aeruginas, S. aureus, E. coli and C. albicans (n = 6) and (C) bacteria growth curves treated with ETGFA containing different silver nanoparticle concentrations (n = 6), (C1) P. aeruginas; (C2) S. aureus; (C3) E. coli and (C4) C. albicans.
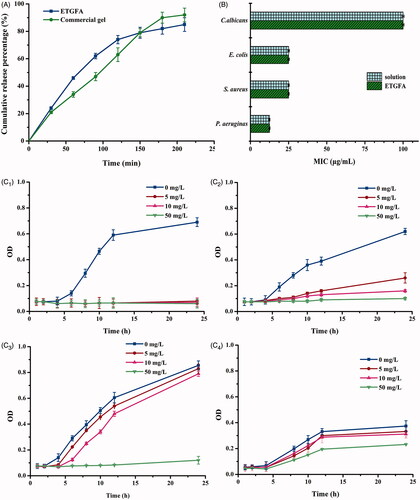
Figure 5. Pathological sections of vaginal, uterine and ovary tissues from rats. (HE staining, ×400). (A) saline; (B) blank ETGFA; (C) silver nanoparticle-loaded ETGFA and (D) silver nanoparticle solution.
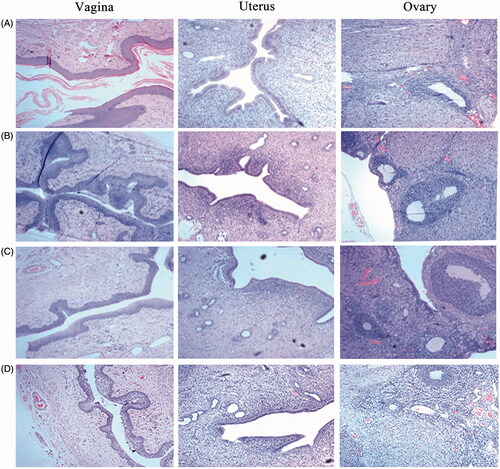
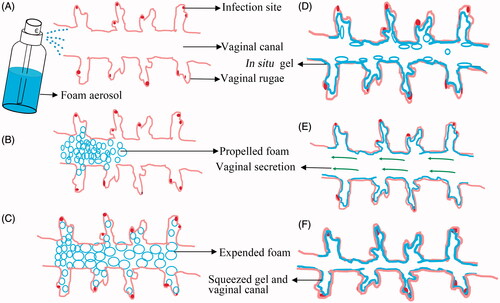
Figure 6. The schematic illustration of the expansible thermal gelling foam aerosol during foam expansion, thermal gelation and gel retention. (A) the ETGFA is administrated as foam aerosol; (B) the foam aerosol is spurted to the vaginal canal by propellant; (C) the foam expands and penetrates to the infectious sites in deep vaginal rugae; (D) the foam thermally transforms into gel to cover the infectious sites on the vaginal mucosa and the gel resists to the self-cleaning during (E) vaginal secretion and (F) vaginal contraction motion.