Figures & data
Table 1. Scoring standard of pathological change in lung tissues.
Figure 1. (a) Schematic of MPS-NSSLs-SPANb nanoparticles. (b) The conjugation was achieved by the reaction between the iodoacetyl group from liposomes and the both imidazolyl side chain nitrogens of histidine functional groups within nanobody. (c) Cryo-TEM images of extruded actively targeted liposomal MPS-NSSLs-SPANb nanoparticles. (d) SDS-PAGE of conjugating SP-A nanobody to liposomes, at a iodoacetate-to-His12 ratio 60:1. 1′ represents SP-A nanobody, 1 represents unpurified MPS-NSSLs-SPANb. (e) ELISA test of SP-A nanobody and different nanoparticles to SP-A antigen. All nanoparticles were purified by gel-filtration to get rid of free nanobody, and fluorescence was quantified at the same level before ELISA test. (f) MPS–NSSLs–SPANb was stored at 4 °C, encapsulation efficiency of MPS–NSSLs–SPANb to MPS was tested every 4 weeks.
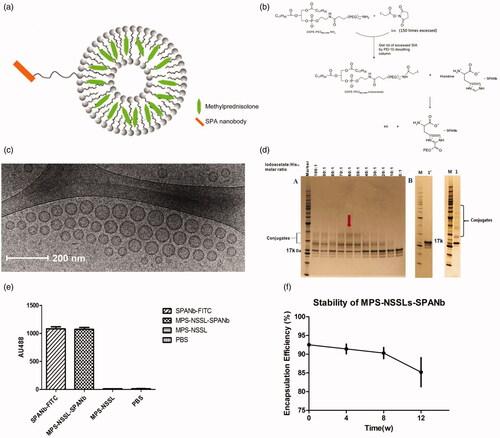
Figure 2. Lung-targeting analysis of MPS-NSSLs-SPANb in nude mice by small animal imaging. The experiments were performed independently three times, and showed similar results.

Figure 3. MPS distribution in plasma and other tissues after injection of MPS, MPS-NSSLs and MPS-NSSLs-SPANb. MPS-NSSLs-SPANb lengthened methyprednisolone’s blood circulation time and increased MPS concentration in both blood and lung tissue.
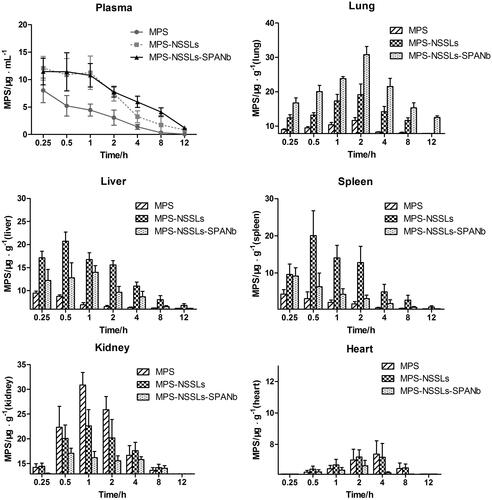
Figure 4. Therapeutic effect of nanoparticles. (a) Lung injury on rats model was highly pathologically improved after treating with MPS-NSSLs-SPANb 1 and 2 weeks later. (b) MPS-NSSLs-SPANb significantly reduced the expression of inflammation cytokine in both BALF and lung tissue.
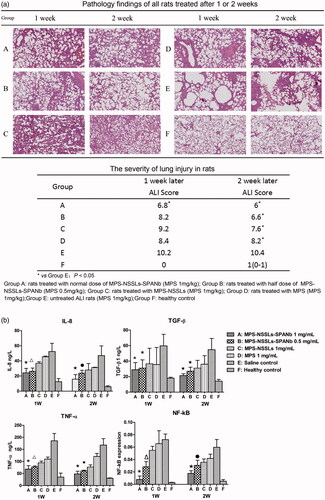
Table 2. Culturing bronchoalveolar lavage fluid to test bacterial and fungal infection.
Table 3. Serum levels of ALT, AST, BUN and Cr in different groups of rats.
