Figures & data
Figure 1. GenJet™ and in vivo-jetPEI enhances the H5N1 vaccine-induced systemic antibody responses and memory B-cell responses. Balb/c mice (5 mice/group) were intranasally administered with 3 µg of A/IN/05 vaccine with or without GenJet™ (10 µl/each mouse as described in previous study (Kulkarni et al., Citation2014)) or in vivo-jetPEI® (0.6 μl/mouse according to manufacturer’s protocol). One month later, mice were boosted with the same vaccine formulations. The control mouse group received GenJet™, in vivo-jetPEI® or PBS at both time points. (A) Three weeks after booster immunization, sera were collected and IgG, IgA and IgM antibodies against A/IN/05 were assessed by ELISA. (B) One week after booster immunization, the spleen were harvested and the frequency of A/IN/05-specific IgG+ ASCs in the spleen were measured by ELISPOT assay. The number of A/IN/05-specific IgG+ ASCs were normalized against the number of total IgG+ secreting ASCs and presented as % Ag-specific IgG+ B cells. The data are representative of two independent experiments (3–5 mice each group) and error bars represent SEM. One-way ANOVA with Bonferroni post-analysis was used to analyze differences among different groups. p < .05, p < .01 and p < .001 as compared to the H5N1 vaccine alone group.
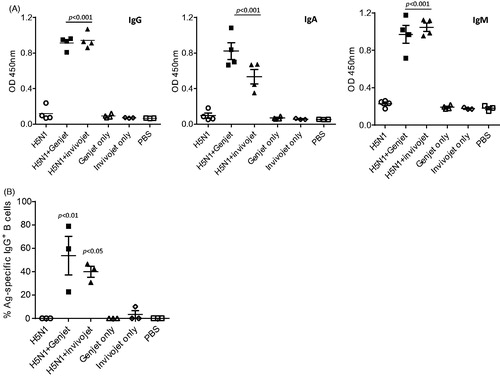
Figure 2. GenJet™ and in vivo-jetPEI® enhanced the H5N1-specific T-cell responses. Balb/c mice (3–5 mice/group) were intranasally administered with A/IN/05 vaccine with or without GenJet™ or in vivo-jetPEI® using prime-boost regimen as described in . (A) One week after booster immunization, the lung tissues were harvested and single cell suspensions were prepared. About 106 cells from the lung were stimulated in vitro with HA peptide for 6 h to examine the antigen-specific CD8 T-cell response; or with RG A/IN/05 virus at an MOI of 1 for 16 h to examine the antigen-specific CD4 T-cell response. GolgiPlug™ was added during the last 5 h of incubation. Cells were surface stained with anti-CD44, anti-CD4 or anti-CD8 antibody (BD Bioscience), followed by intracellular staining with anti-IFNγ antibody (BD Bioscience). The frequency of IFN-γ producing T cells in total activated T cells was presented. (B) One-week post-booster immunization, the draining lymph nodes, lungs and spleen tissues were harvested and the frequency of HA518-specific CD8 T cells in total activated CD8 T cells was stained using H-2Kd/IYSTVASSL tetramer. (C) Three weeks after booster immunization, sera were collected and IgG2a, IgG2b IgG3 and IgG1 antibodies against A/IN/05 were assessed by ELISA. The data are representative of two independent experiments (3–5 mice each group) and error bars represent SEM. One-way ANOVA with Bonferroni post-analysis was used to analyze differences among treatments. p < .05, p < .01 and p < .001 as compared to the H5N1 vaccine alone group.
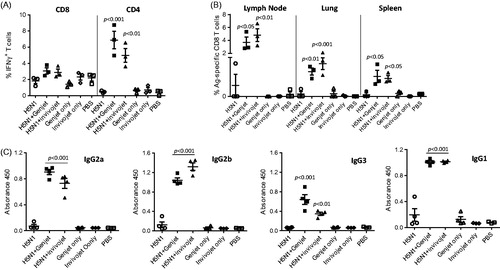
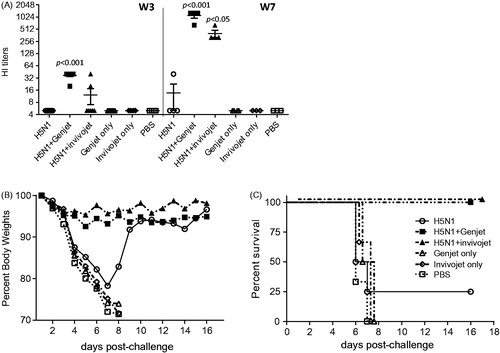
Figure 3. GenJet™ and in vivo-jetPEI enhanced the protective immunity. Balb/c mice (5 mice/group) were intranasally administered with A/IN/05 vaccine with or without GenJet™ or in vivo-jetPEI® using the prime-boost regimen as described in . (A) Sera were collected at the third week following primary immunization (W3) and booster immunization (W7). HI titers were measured against RG A/IN/05 virus. (b,c) Four weeks following booster immunization, mice were challenged with 5 × LD50 of RG A/IN/05 virus. Mice were weighed every day to monitor body weight changes (B) and mortality (C). Mice that lost more than 25% body weight were euthanized and scored as a fatality. One-way ANOVA with Bonferroni post-analysis was used to compare the percentage of body weight changes and the log-rank (Mantel–Cox) test was used to compare percent survival among groups of mice. n = 5 mice for each group from two independent experiments and the error bars represent SEM. p < .05, p < .01 and p < .001 as compared to the H5N1 vaccine alone group.