Figures & data
Figure 1. (a) H&E staining of dosed lung sections for morphological evaluation. (b) Distribution of salmeterol at 5, 15, 30, and 60 min after administration via nebulization using MALDI-MSI at 70 µm spatial resolution (duplicate biological replicates). White denotes the maximal signal intensity per image and black the minimal signal intensity observed. (c) Kinetic profile of salmeterol levels with overlay of its relative abundance from MSI (light grey) and its concentration from lung homogenate bioanalysis (dark grey). Intensity scale 0–100% Scale bar = 6 mm.
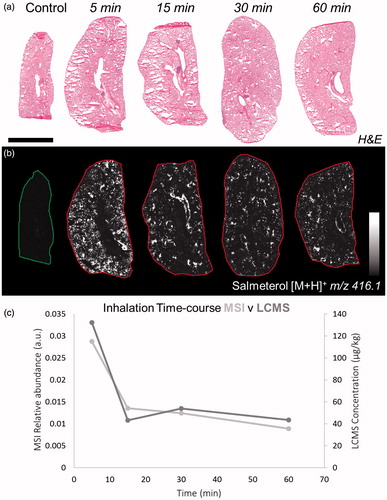
Figure 2. (a) H&E staining of inhaled and IV dosed lung sections. (b) MALDI images of corresponding tissues at 35 µm spatial resolution showing the differential distribution of salmeterol depending on administration route (duplicate biological and technical replicates). Inhaled salmeterol was preferably retained in bronchioles whereas systemic dose was more widely detected within tissue 30 min after delivery. (c and d) Higher magnification of a bronchiolar area in both conditions, the different administration routes clearly display differential distribution. Intensity scale 0–100%. Scale bars = 5 mm (black)/1 mm (white).
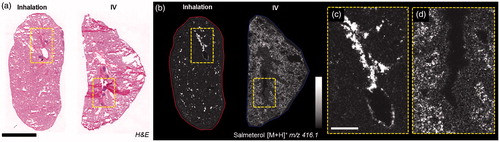
Figure 3. (a) H&E staining of control and dually dosed (salmeterol for inhalation and d3-salmetrol for IV) lung sections. (b and c) Salmeterol (inhaled) and d3-salmeterol (IV) distributions using MSI at 50 µm of spatial resolution showing the differential localization of both administered compounds (duplicate biological and triplicate technical replicates) at 30 min after delivery. Intensity scale 0–100% for both salmeterol versions. Scale bar = 1.2 mm. (d) Evaluation of salmeterol distribution heterogeneity depending on administration route (IV versus Inhaled) based on pixel-to-pixel relative standard deviation (RSD in %) in the whole lung image. A higher heterogeneity of the salmeterol signal is observed for inhaled compound than IV dosed (214% versus 75% in median, n = 6).
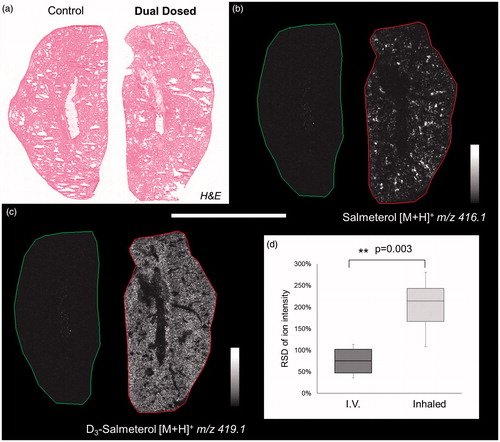
Table 1. Spatial correlation between tissue endogenous marker (blood vessels, alveolar, and bronchiolar) and inhaled (salmeterol) and IV (d3-salmeterol) compound distributions.
Figure 4. (a) H&E from dual dosed lung section focus on analyzed region. (b) Spatial clustering of high spatial resolution (10 µm) MSI of corresponding region showing similarities between tissue compartment molecular composition. (c) Region of Interest (ROI) generation from segmentation results corresponding to three tissue sub-structures (triplicate biological replicates). MS Images of inhaled (d), IV administered salmeterol (e), and overlay of both distributions (f). Data shows discrete localization of inhaled salmeterol to epithelium and sub-epithelium compared to IV dosed salmeterol within alveolar regions at 30 min after delivery. Intensity scale 0–100% for both salmeterol versions. Scale bar = 600 µm.
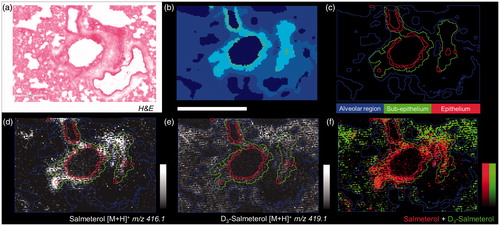
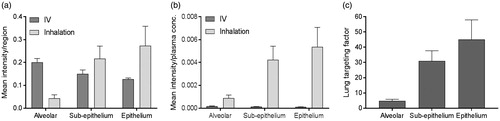
Figure 5. (a) Histogram of the mean intensity of salmeterol determined by MSI per segmented region, i.e. alveolar, sup-epithelium and epithelium, after inhalation or systemic dosing (n = 3) at 30 min after delivery. (b) The mean intensity in each region was normalized against the plasma concentration in respective administration group (n = 3). (c) The targeting factor is the ratio between inhalation and IV group in (b), in respective region, and it describes the gain with inhalation over systemic dosing at equivalent plasma concentrations.