Figures & data
Table 1. 23 full factorial design layout for formulation of GBP rafts: factors and responses.
Table 2. Multiple reaction monitoring (MRM) conditions.
Figure 1. Light photos showing floating behavior of optimized GBP raft formula at different time intervals in 0.1 N HCl.
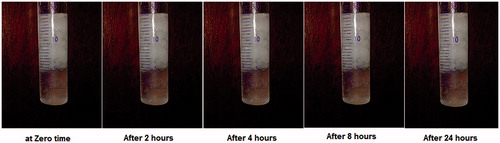
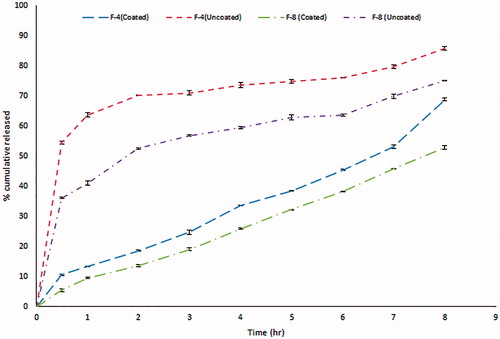
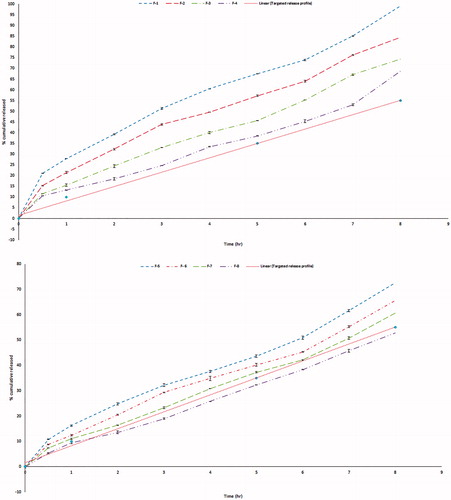
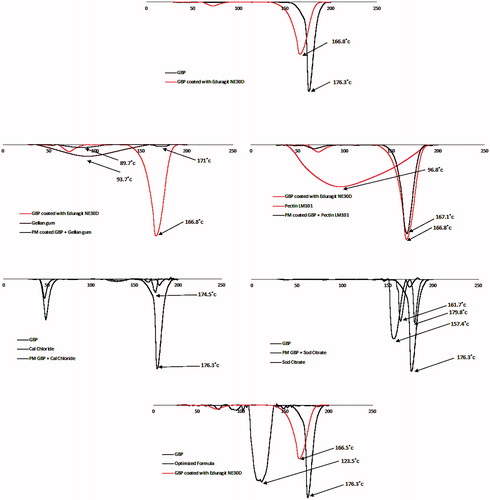
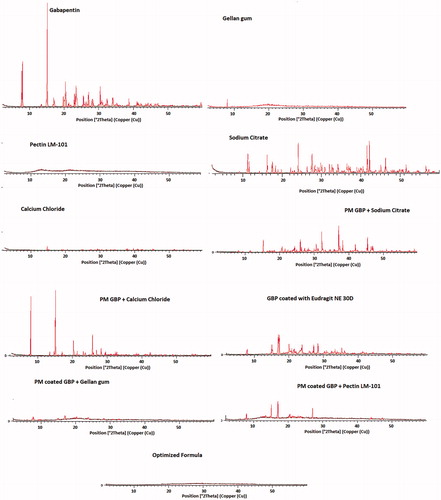
Table 3. Composition of the prepared GBP formulations in actual values (un-coded units) and their observed responses.
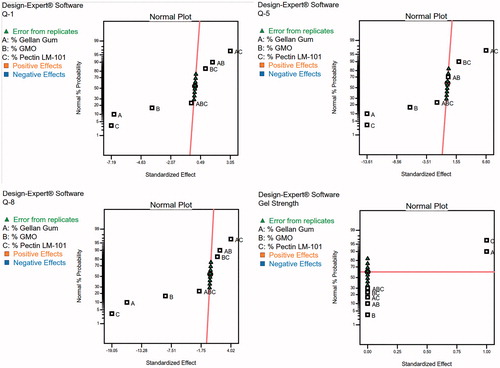
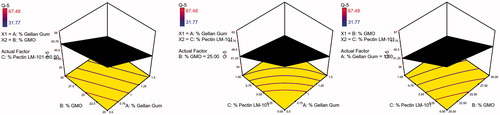
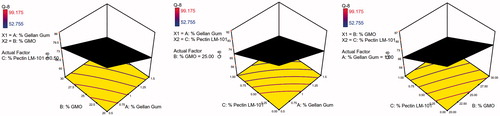
Table 4. Coefficient estimates for different model terms appearing in the final equation for each response and their significance levels.
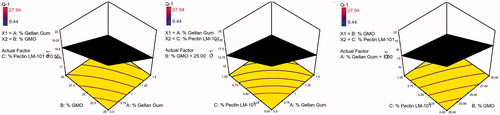
Table 5. Coefficient estimates of the fitted regression model in terms of coded factors for the responses and the corresponding R2 values.
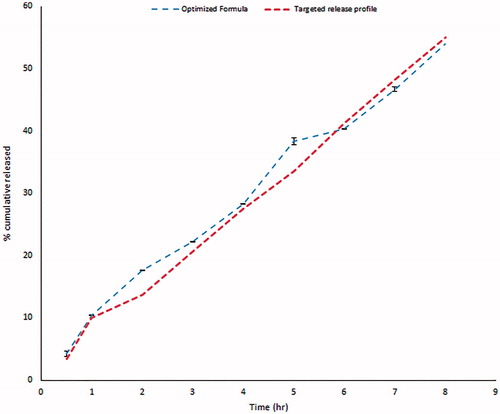
Table 6. The observed and predicted values for the optimized GBP raft formulation.
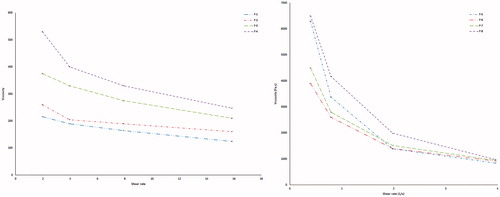
Table 7. Rheological behavior of different formulae in both sol and gel forms.
Figure 13. Light photos showing presence of gels in rat stomach at different time intervals after oral administration of optimized formula.
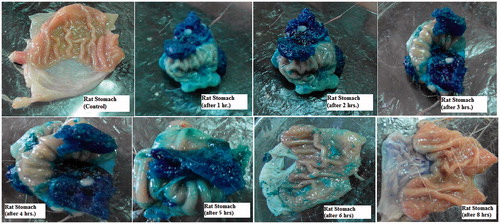
Figure 14. Average plasma concentration time profiles after single oral administration of both GBP raft forming systems and immediate release marketed Neurontin® oral solution to six human volunteers.
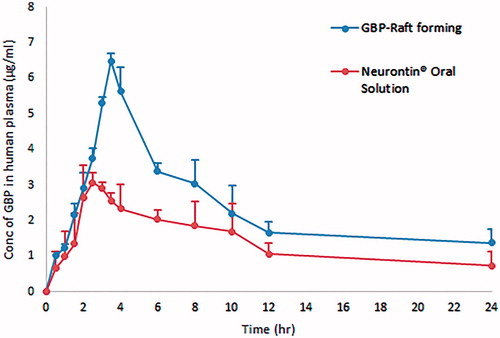
Table 8. Average pharmacokinetic parameters of both GBP raft forming systems and immediate release marketed Neurontin® oral solution following the administration to six human volunteers.