Figures & data
Figure 1. GSH-activatable response of Biotin-ss-CPT. (A) Proposed mechanism in the targeted delivery system (Biotin-ss-CPT) and the CPT activation by GSH. (B) Reverse-phase HPLC chromatograms of Biotin-ss-CPT with GSH. Peaks in the chromatograms were detected by monitoring the UV/Vis absorption at 365 nm. HPLC: Agilent 1260 Infinity II system, Agilent ZORBAX SB C18 (Santa Clara, CA, USA) (250*4.6 mm, 5 µm) Mobile phase A: H2O, B: CH3CN. 0–20 min: 50–100% B. Flow rate: 1 mL/min. (C) HRMS spectrum of the products from the reaction of Biotin-ss-CPT (20 µM) in a mixed solution of PBS buffer and DMSO (v/v: 4/1) with two equivalent of GSH. Spectrum was obtained 12 h after incubation at 37 °C. The pH dependence at 5.3 (D), 6.8 (E) and 7.4 (F) on the fluorescence spectra variation of Biotin-ss-CPT in the presence of GSH (8 equiv) and the variation in fluorescence intensity at 430 nm (G) before and after incubation with GSH. The data were recorded every 30 min for 2 h after incubation with GSH in mixed solution of PBS buffer and DMSO (v/v: 4/1). Excitation was set at 365 nm.
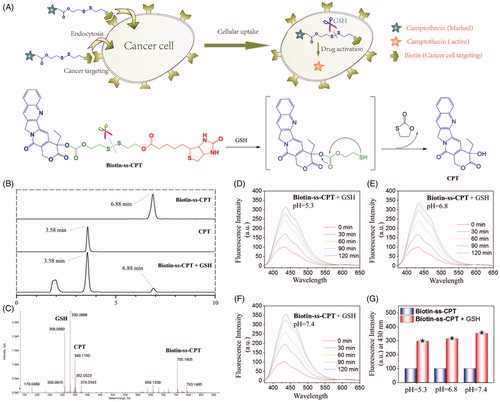
Table 1. In vitro cytotoxic activity IC50 (μM) of Biotin-cc-CPT, Biotin-ss-CPT, and CPT.
Figure 2. (A) Intracellular uptake of CPT and biotin-conjugates on tumor cells (MGC 803 and SW620) and corresponding normal cells (GS1 and NMC460) during of 8-h period. Error bars represent SD of n = 3 data sets. (B) Inhibitory effects of migration of Biotin-ss-CPT, Biotin-cc-CPT and free CPT on MGC 803 cell lines. Cells were treated with 1 μM biotin-conjugated CPTs or free CPT for 24 h then Nuclei (blue) were stained with DAPI. (C) Flow cytometric analysis of MGC 803 cell cycle distribution. (D) The proportional (%) of Sub-G1 phase population. (E) The activity of caspase 8, 9 and 3 on MGC 803 cells after treatment with biotin-conjugated CPTs. MGC803 cells were treated with CPTs at 0.5, 1, and 2 μM for 48 h.
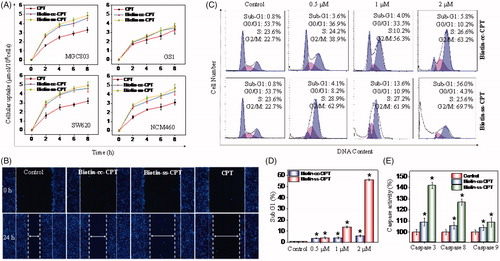
Figure 3. Induction of ROS-mediated mitochondrial dysfunction and perturbation of GSH/GPXs system. (A) Photomicrographs of mitochondria fission and cytoplasmic shrinkage induced by 1 μM biotin-conjugated CPTs as detected using Mitotracker & DAPI co-staining. The state of mitochondrial fission is indicated by the arrows. (B) Flow cytometric analysis of the changes in ΔΨm on MGC 803 cells treated with Biotin-cc-CPT or Biotin-ss-CPT. The proportion in the right region emitting green fluorescence stands for the percentage of cells that due to the loss of ΔΨm. (C) ROS generation triggered by biotin-conjugated CPTs on MGC 803 cells. Cells incubated with 10 μM DEH in PBS for 30 min were exposed to biotin-conjugated CPTs. (D) ROS generation marked by 10 μM DCF fluorescence. Cells treated with 1 μM of biotin-conjugated CPTs. (E) and (F) Changes in intracellular GSH level and GPXs activity. Cells were treated with 1 μM of biotin-conjugated CPTs. .01 < p ≤ .05 and p ≤ .01 are considered to be statistically significant and highly significant and are denoted as “*” and “**”, respectively. Student’s t test.
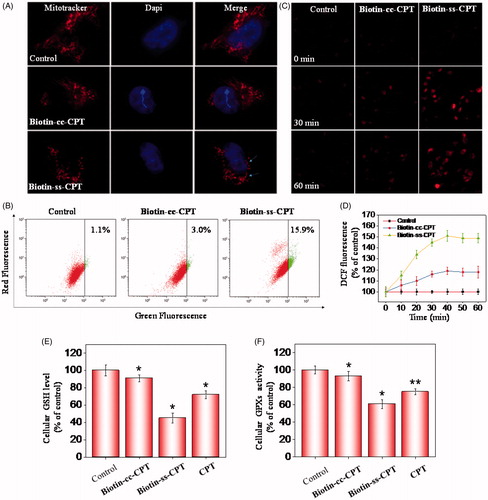
Figure 4. In vivo anticancer activity. (A) Changes in tumor volume of different drug-treated MGC803 cancer nude mice for 22 days. (B) Body weight of xenograft MGC 803 cancer nude mice treated by different drugs. (C) Relative tumor growth ratio. (D) H & E stained tissue sections from tumor of the mouse treatment of different drugs. (E) T2 MR images of tumors treated with different drugs after 22-day drug injection. (F) Pseudo-color images of the ADCs.
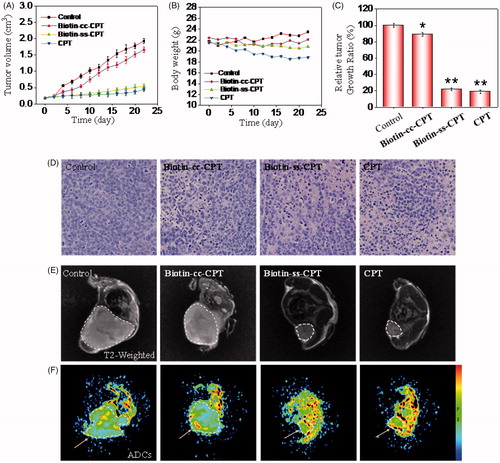
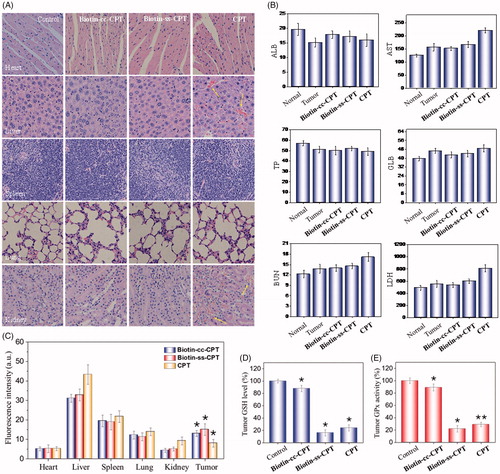
Figure 5. (A) H & E staining section of heart, liver, spleen, lung, kidney from nude mice after treatment with equivalent dose of 2 mg/kg CPT after 22 days treatment. (B) Blood biochemistry analysis of the indices of ALB, AST, TP, GLB, BUN, and LDH in mice. (C) In vivo biodistribution of different drugs in major organs. (D) Tumor GSH level. (E) Tumor GPx activity. *p < .05, **p < .01. Student’s t test.