Figures & data
Figure 1. (A) Setup of in vitro release of celecoxib (CXB). Absolute (B) and cumulative (C) celecoxib release from PEA microspheres (PEAMs) in plain culture medium: after 28 days, 40% was released. N = 2 per time point. (D) CXB release in plasma after intra-articular injection in osteoarthritic rat knee joints with three different loadings as indicated. N = 6 per group. Plasma CXB concentrations were significantly different (p < .001) between groups at all timepoints, except for T = 144h. Data depicted as average ± SD.
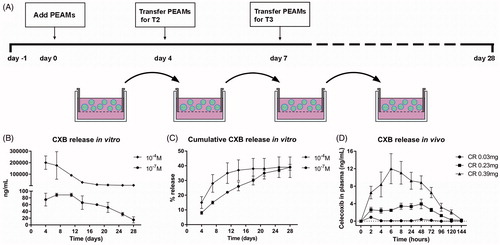
Table 1. Overview of experimental groups and microsphere loading.
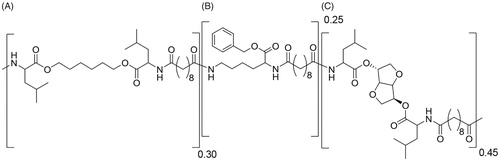
Table 2. Polymer characterization.
Figure 3. (A) Setup of the pressure plate measurements. (B) Body weight increased gradually in all groups during the course of the study. (C) Load bearing significantly decreased (p = .013) in operated joints 3 weeks after anterior cruciate ligament transection and partial medial meniscectomy (ACLT + pMMx). (D) Static weight bearing improved 6 weeks after OA induction, but only the LD-CXB-PEAMs could significantly enhanced weight bearing compared to OA control joints (p = .044). ACLT: Anterior cruciate ligament transection; OA: osteoarthritis (unloaded PEAM control group); LD CXB: low dose celecoxib; MD CXB: middle dose CXB; HD CXB: high dose CXB.
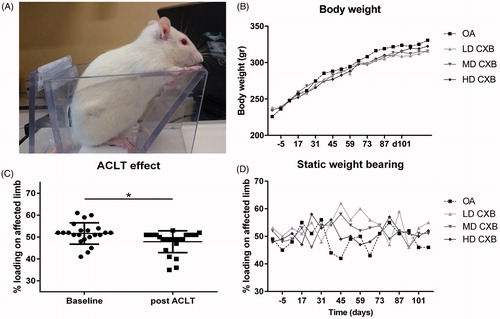
Figure 4. Ex vivo micro computed tomography (µ-CT) analysis of the medial tibia plateau. The induction of osteoarthritis (OA) led to an increase in subchondral sclerosis (A,B; p < .001), which was inhibited by the controlled release (CR) of celecoxib (CXB) (A; p = .007, p = .063, p = .003 for low, middle and high dose CXB (LD-, MD- and HD-CXB)). CXB-PEAMs exerted a protective effect on the formation of osteophytes (C,D; p = .006, p = .036 and p = .019 for LD-, MD- and HD-CXB) and the presence of loose bodies (E,F; p = .011, p = .24, p = .142) in OA joints. CR of celecoxib reduced the number of subchondral bone cysts (G,H; LD-CXB p = .002; MD-CXB p = .037; HD-CXB p = .002) in OA joints, and also resulted in smaller cysts (I; p = .0015, p = .002, p = .0018). The induction of OA resulted in increased bone volume of the subchondral bone plate (J; p = .059, ES 0.7), which was decreased by HD-CXB-PEAMs (p = .09, ES 0.8). Bone volume of the trabecular bone was decreased after OA induction (K, p = .027), but was not influenced by CXB-PEAMs. HD-CXB-PEAMs tended to prevent increase of trabecular bone spacing (K; p = .063, ES 0.65). *p < .05, **p < .01, a: medium effect size (ES); b: large ES; c: very large ES.
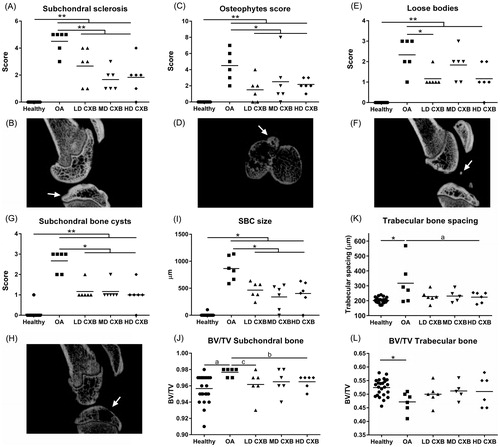
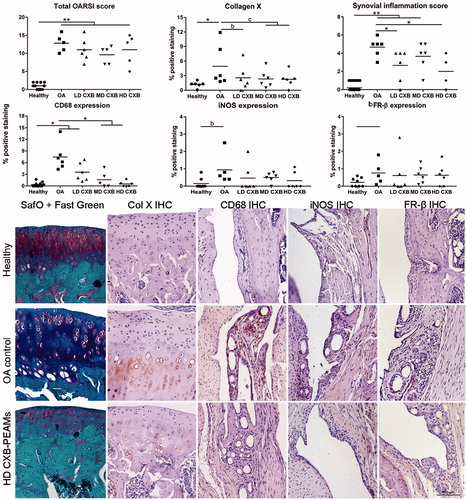
Figure 5. Histological grading and immunohistochemical analysis of the medial tibial. OA induction led to an increase in OARSI score, collagen X deposition and synovial inflammation. Celecoxib-loaded PEAMs were able to decrease synovial inflammation, macrophage presence and collagen X deposition. *p < .05; **p < .01; a: medium effect size (ES); b: large ES; c: very large ES. OA: osteoarthritic control knees; LD: low dose; MD: middle dose; HD: high dose celecoxib. HD CXB-PEAMs: high dose celecoxib-polyesteramide microspheres; IHC: immunohistochemistry.