Figures & data
Table 1. Composition of VP-loaded polymeric beads adopting D-optimal design with their resultant dependent variables.
Figure 1. SEM images showing (A) the prepared superamphiphobic substrate with micro-length hierarchical structure at different magnification powers (800, 3000, and 6000 x, respectively) and (B) the optimized VP-loaded PCL based bead system (“OS”) with different magnification powers (120× and 1000×).
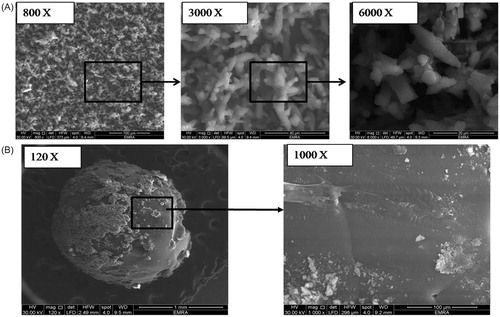
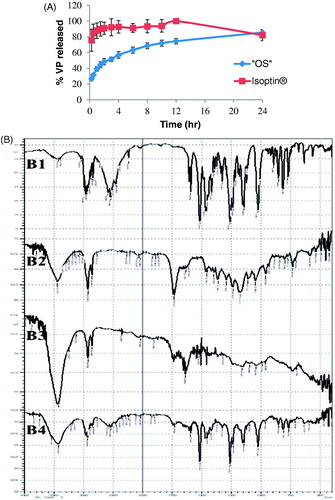
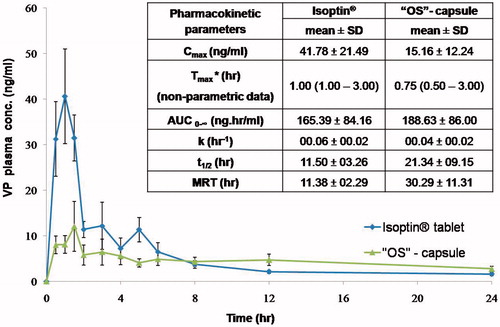
Figure 2. (A) Contact angles and percentages of the trapped air for different liquids on the superamphiphobic treated aluminum surface compared to the contact angles of the untreated surface; While (B) optical images of contact angles for water (B1), olive oil (B2), and the optimized VP-loaded PCL based solution (B3).
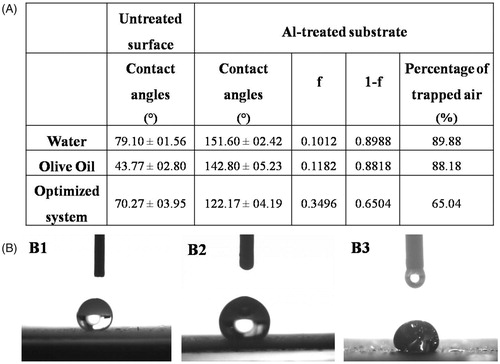
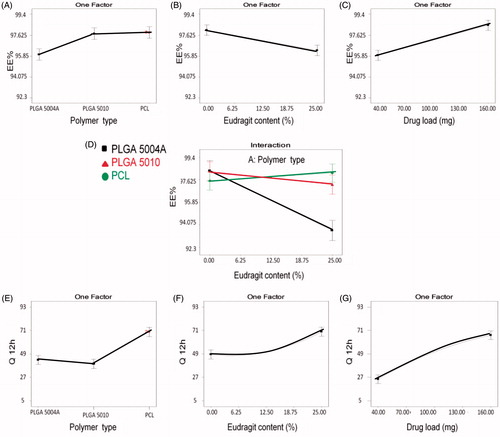
Figure 3. Line plot presenting the effect of the variables on: entrapment efficiency (EE%): Effect of (A) main polymer type, (B) Eudragit RS100 content, (C) drug load, and (D) two factor interaction between polymer type and Eudragit RS100 content. In-vitro release (Q12hr): Effect of (E) main polymer type, (F) Eudragit RS100 content, and (G) drug load.