Figures & data
Figure 1. Anatomy of the small intestine and the implications for orally applied nanocarriers. Multiple consecutive close-ups are displayed. In order to enter the bloodstream, orally applied nanocarriers have to cross multiple borders of the human body. Especially the uptake and crossing of enterocytes in the small intestine mark a key challenge in oral drug delivery via nanocarriers.
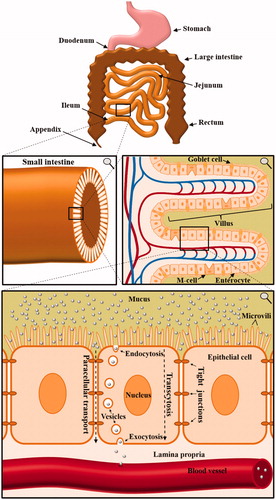
Figure 2. Close-up of nanoparticles trapped in late endosomes/lysosomes. In this study, Caco-2 cells were incubated with 500 µg/mL polystyrene nanoparticles for 24 hours. The addition resulted in huge endolysosomal structures up to 2 µm in diameter as analyzed by (A) confocal laser scanning microscopy (scale bar =10 µm) and (B) transmission electron microscopy (Reinholz et al., Citation2018). Reprinted from J. Reinholz, C. Diesler, S. Schöttler, M. Kokkinopoulou, S. Ritz, K. Landfester, V. Mailänder, Protein Machineries defining Pathways of Nanocarrier Exocytosis and Transcytosis, Acta Biomaterialia, Copyright (2018) with permission from Elsevier.
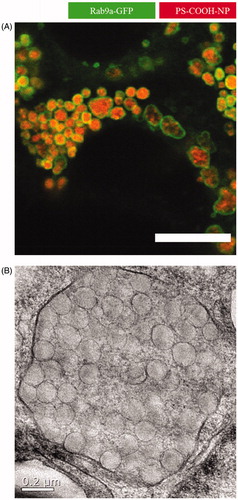
Table 1. Uptake mechanisms of various nanomaterials in endo- and epithelial cell lines.
Figure 3. Transcytosis of 12 nm human serum albumin–gold nanoparticles across a hCMEC/D3 endothelial cell monolayer. In this study, the authors demonstrated a protocol for the in vitro determination of nanoparticle translocation across a cell monolayer using transmission electron microscopy. (a) Nanoparticles present in apical sorting vesicles within the cytosolic space. (b) Gold nanoparticles detected in different cytoplasmic regions. (c) Nanoparticles co-localizing with endosomes. (d) Nanoparticles co-localizing with lysosomes. (e) A single gold nanoparticle exits the basolateral cell membrane from a vesicle. (f) The nanoparticle is finally present in the basal compartment. EP: endocytic pit; ER: endoplasmic reticulum; EV: exocytic vesicle; E: endosome; L: lysosome; M: mitochondria; N: nucleus; TJ: tight junction (Ye et al., Citation2015). Reproduced from D. Ye, K.A. Dawson, I. Lynch, A TEM protocol for quality assurance of in vitro cellular barrier models and its application to the assessment of nanoparticle transport mechanisms across barriers, Analyst 140(1) (2015) 83-97 with permission of The Royal Society of Chemistry.
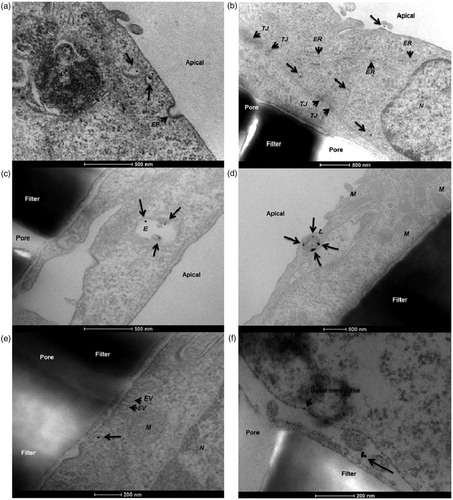
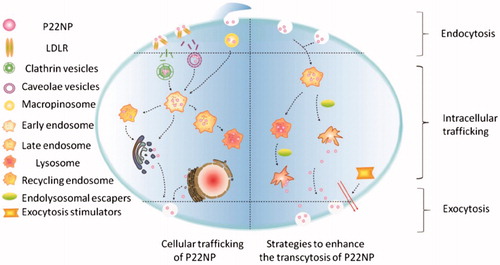
Figure 4. Putative pathways for endolysosomal escape and exocytosis of P22NPs in Caco-2 cells. The addition of hemagglutinin-2 and metformin led to an increase in endolysosomal escape and exocytosis of nanoparticles, overall resulting in an increase in transcytosis. The black arrows represent the pathways that were demonstrated by the authors (Cui et al., Citation2018). Reproduced from Y. Cui, W. Shan, R. Zhou, M. Liu, L. Wu, Q. Guo, Y. Zheng, J. Wu, Y. Huang, The combination of endolysosomal escape and basolateral stimulation to overcome the difficulties of ‘easy uptake hard transcytosis’ of ligand-modified nanoparticles in oral drug delivery, Nanoscale (2018) with permission of The Royal Society of Chemistry.