Figures & data
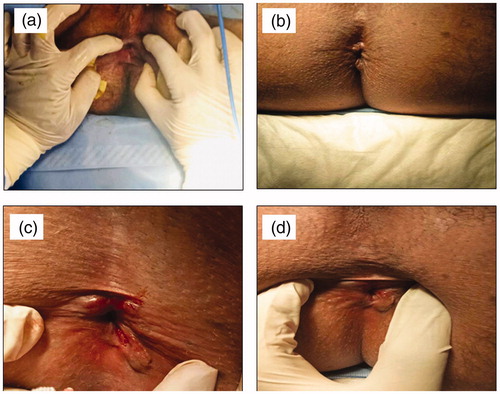
Figure 1. (a–e) DSC thermograms of LDH, NIF and BMV as (a) drug–drug mixtures where (I) LDH (II) NIF (III) BMV (IV) LDH-NIF (V) LDH-BMV (VI) NIF-BMV (b) tertiary drug mixture, (c) LDH-excipient mixtures, (d) NIF-excipient mixtures, and (e) BMV-excipient mixtures; where I) drug alone, II) drug-CP940, III) drug-P407, IV) drug-methylparaben, V) drug-propylparaben, and VI) drug-titanium-dioxide. (f–h) FT-IR spectra of (f) LDH-P407, (g) LDH-methylparaben, (h) LDH-propylparaben, as control sample (I) and sample subjected to isothermal stress (II).
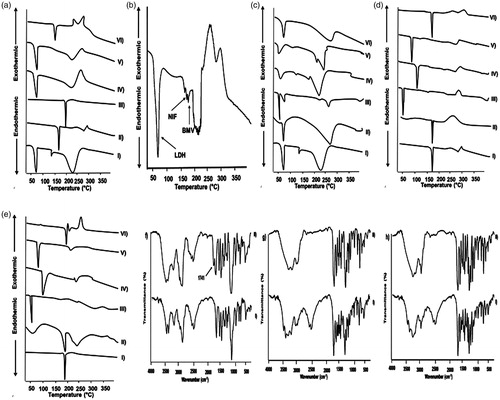
Table 1. (a) Peak temperature (Tpeak) values of drugs, drug–drug and drug-excipient mixtures (1:1 w/w). (b) IST results of drugs, drug–drug, and drug-excipient mixtures (1:1 w/w) after 4 weeks of storage at stressed conditions.
Figure 2. (a) Effects of irradiation on NIF photostability in gel formulations containing different concentrations of titanium dioxide. (b–d) In vitro release of (b) NIF, (c) LDH, and (d) BMV in phosphate buffer pH 7.4.
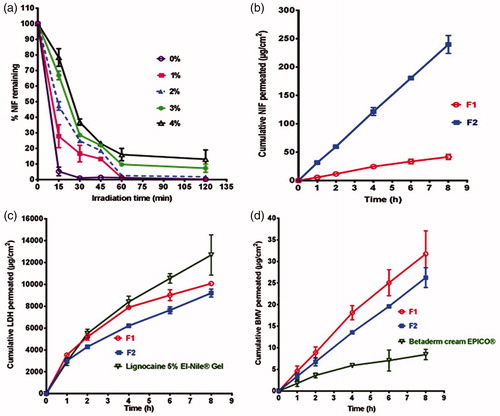
Table 2. In vitro evaluation of the prepared combination gels (F1 and F2) and the market products (a) Viscosity, pH, drug content (%), and steady-state flux (Jss). (b) Kinetic modeling of release data. (c) Stability study data of the optimized combination gel (F2) after storage for three months at different temperatures.
Table 3. Clinical study (a) Baseline characteristics of the study population. (b) Prevalence of fissure healing, pain, bleeding, discharge, and itching among patients with AAF and CAF at first, third, and sixth week post-treatment.
Figure 3. Representative photographs of patients suffering from either AAF (a) before treatment (baseline), (b) after treatment for six weeks with the optimized combination gel (F2) or CAF, (c) before treatment (baseline), and (d) after treatment for six weeks with the optimized combination gel (F2).