Figures & data
Table 1. Composition of NLC formulations prepared with Precirol®ATO5 as solid lipid, with Tween®80 (NLC1 serial code) or Pluronic®F68 (NLC2 serial code) as Surfactant, and different liquid lipid (Transcutol (A), oleic acid (B) and castor oil (C)), both empty and loaded with hydrochlorothiazide (HCT).
Table 2. Mean particle size, polydispersity index (PDI), zeta potential (ζ), encapsulation efficiency (EE%) and loading capacity (LC%) of different NLC formulations (see for their composition).
Figure 1. HCT in vitro release profiles from drug aqueous suspension or from Tween®80- and Pluronic®F68-based NLC formulations containing ricin oil as liquid lipid (NLC1C and NLC2C) prepared by the homogenization-ultrasonication method.
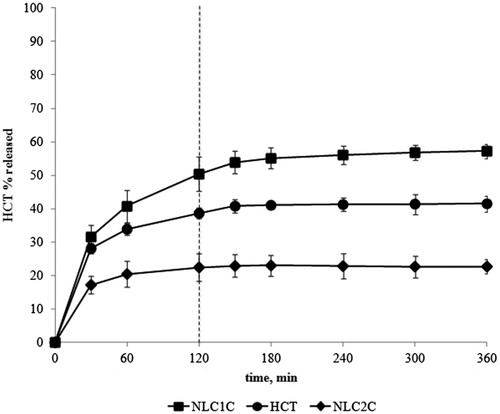
Table 3. Mean particle size, polydispersity index (PDI), zeta potential (ζ), Encapsulation Efficiency (EE%) and Loading Capacity (LC%) of different ME-based NLC formulations.
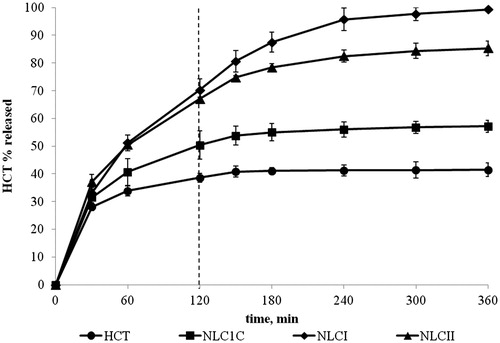
Figure 2. HCT in vitro release profiles from NLC formulations produced by the microemulsion method containing S:Co-S mixtures of Tween®80:Tween®20 at 1:4 w/w ratio (NLCI) and Tween®80:Solutol®HS at 1:1 w/w ratio (NLCII) in comparison with the corresponding Tween®80-based NLC formulations prepared by the homogenization-ultrasonication method (NLC1C) and simple aqueous suspension.