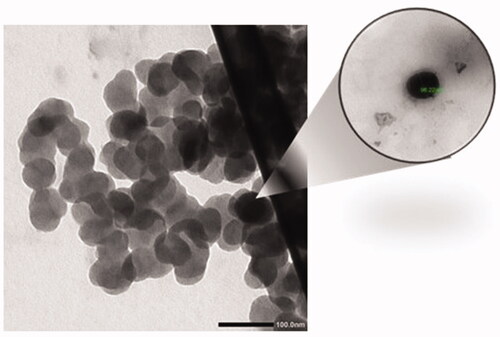
Figures & data
Table 1. Compositions of NLC formulations.
Table 2. 24 full factorial design variables and levels of NLC-cinnamon oil.
Table 3. Antibiogram of used multidrug resistant P. aeruginosa strains.
Table 4. The factorial design formulation matrix (n = 3).
Figure 1. 3D plot model graphs demonstrating the effect of factors. (A) Type of solid lipid, (B) solid:liquid lipid ratio, (C) total lipid content, and (D) SAA concentration on mean particle size (nm).
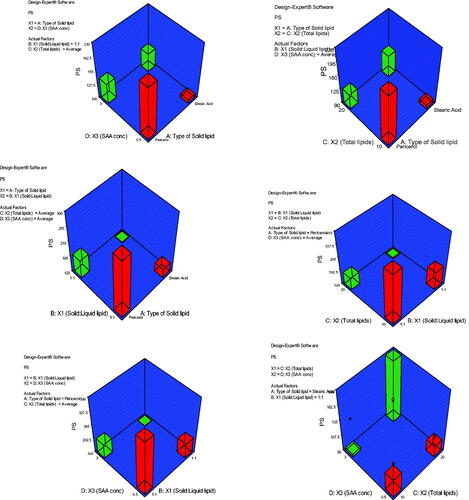
Figure 2. 3D plot model graphs demonstrating the effect of factors. (A) Type of solid lipid, (B) solid:liquid lipid ratio, (C) total lipid content, and (D) SAA concentration on mean zeta potential (mV).
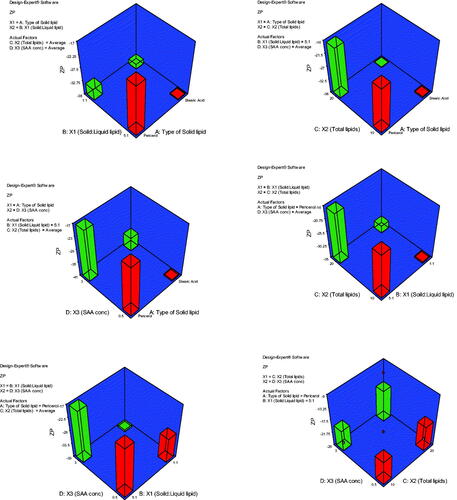
Figure 4. FTIR band spectra of cinnamon oil, stearic acid, Labrafac, Tween 80, and the chosen NLC-optimized formulation.
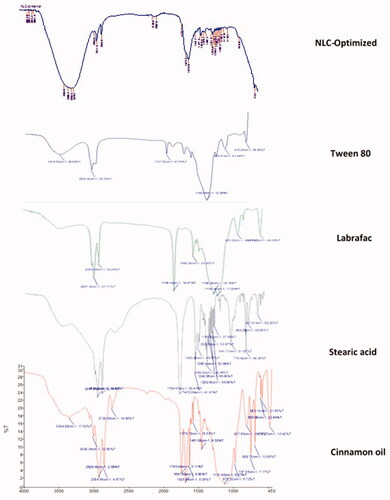
Table 5. Comparison of the spreadability and viscosity of pure gel, blank NLC gel, and NLC-cinnamon oil gel (values are expressed as mean ± 1 SD, n = 3).
Figure 5. In vitro release of cinnamon oil from NLC colloid and gel compared to pure cinnamon oil gel in phosphate buffer pH 7.4 containing 30% absolute ethanol at 35 ± 0.5 °C (n = 3).
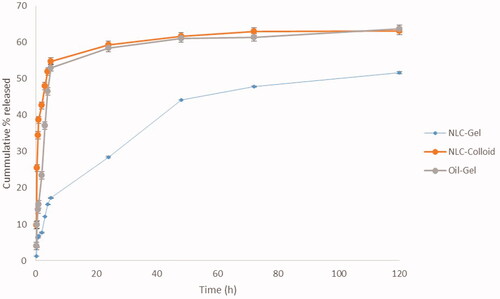
Table 6. Modeling of drug release from NLC-cinnamon gel, NLC-cinnamon colloid, and cinnamon oil-gel formulations.
Table 7. Comparison of MIC against five strains of P. aeruginosa in formulation and excipients.
Figure 6. Images of original burn wound (day 0) and after treatment on day 6. Group 1: no treatment, group 2: cinnamon oil, group 3: NLC-cinnamon oil colloid, group 4: NLC blank gel, and group 5: NLC-cinnamon oil gel.
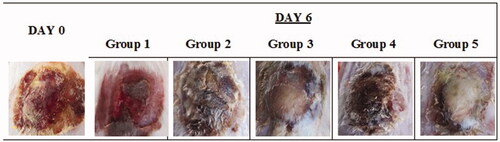
Figure 7. Weight changes during the treatment. Group 1: no treatment, group 2: cinnamon oil, group 3: NLC-cinnamon oil colloid, group 4: NLC blank gel, and group 5: NLC-cinnamon oil gel.
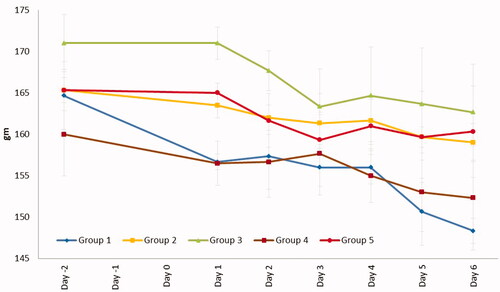
Figure 8. Burn area contraction (cm2) of tested groups. Group 1: no treatment, group 2: cinnamon oil, group 3: NLC-cinnamon oil colloid, group 4: NLC blank gel, and group 5: NLC-cinnamon oil gel (p<.0001).
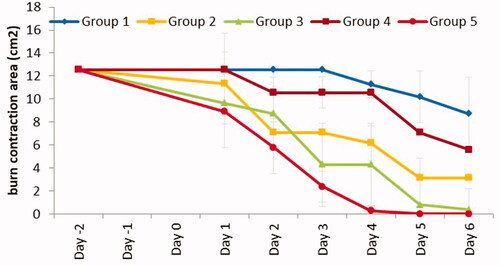
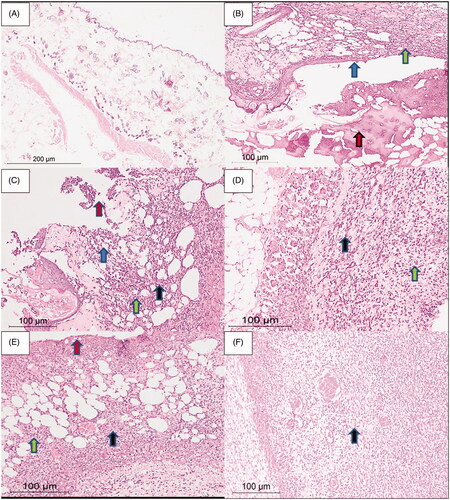
Figure 9. Rat skin samples after the six-day treatment. (A) The normal skin control group showed intact skin surface epithelium and normal dermal and subcutaneous tissues without inflammation (H&E, ×100). (B) Group 1 – no treatment: wide ulceration of the surface (blue arrow) covered by an excessive amount of fibrinoid material (red arrow). The dermis and the subcutaneous tissue show mixed acute and chronic inflammatory cells (green arrow), H&E ×200. (C) Group 2 – cinnamon oil gel: smaller skin ulcers (blue arrow) covered by fibrinoid material (red arrow). The dermis and subcutaneous tissue show inflammatory infiltrate (green arrow) and fibrosis (black arrow) H&E ×200. (D) Group 3 – NLC-cinnamon oil colloid: wide areas of granulation tissue (green arrow) admixed with fibrosis denoting the healing process (black arrow) H&E ×200. (E) Group 4 – NLC blank gel: focal ulcerated area covered by fibrinoid material (red arrow) with small areas of granulation tissue and fibrosis (black arrow) extending to the dermis and subcutaneous tissue with moderate mixed acute and chronic inflammatory infiltrate (green arrow), H&E ×200. (F) Group 5 – NLC-cinnamon oil gel: wide areas of granulation tissue and fibrosis occupying the whole dermis (black arrow), H&E ×200.