Figures & data
Table 1. Various independent and dependent variables used in the Box–Behnken design for the preparation of thymoquinone encapsulated chitosan modified polycaprolactone nanoparticles (THQ-CPLNPs).
Table 2. Observed Box–Behnken experimental runs of thymoquinone encapsulated chitosan modified polycaprolactone nanoparticles (THQ-CPLNPs) with their experimental value.
Figure 1. Effect of independent variables polycaprolactone, chitosan and polyvinyl alcohol on (A). size, (B). PDI, and (C). encapsulation efficiency.
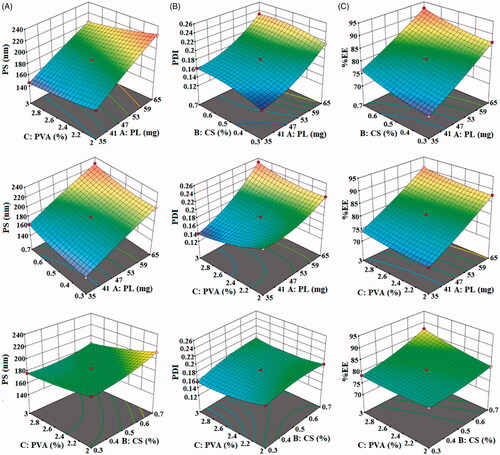
Table 3. Summary of regression analysis for particle size (PS; Y1), polydispersity index (PDI; Y2), and entrapment efficiency (EE; Y3) for fitting data to different models.
Table 4. Analysis of variance of response surface quadratic model of each response.
Figure 2. Predicted and actual value graph of the (A). particle size; (B). PDI; (C). encapsulation efficiency.
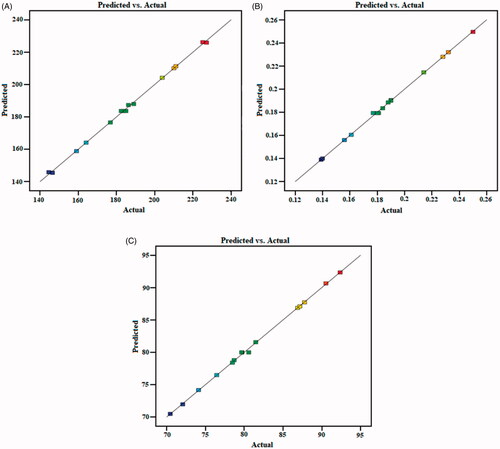
Figure 3. Particle size (A). Zeta potential (B) of optimized thymoquinone chitosan-polycaprolactone nanoparticles (THQ-CPLNPs)..
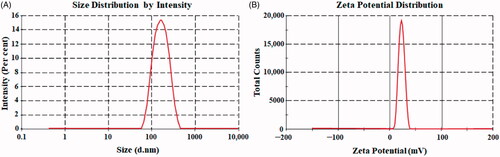
Figure 4. Thymoquinone suspension (THQ-S) and optimized thymoquinone chitosan-polycaprolactone nanoparticles (THQ-CPLNPs). (A) 0.1 N HCl, (B) Phosphate buffer saline.
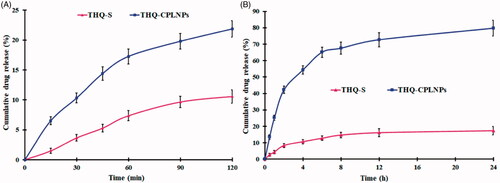
Table 5. Release kinetic models fitting in terms of linear regression coefficient (R2).
Figure 5. Thymoquinone suspension (THQ-S) and optimized thymoquinone chitosan-polycaprolactone nanoparticles (THQ-CPLNPs). (A) THQ permeated, (B) THQ transported.
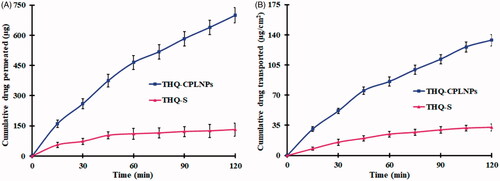
Figure 6. Permeation depth image (A). Thymoquinone suspension (THQ-S) (B). Optimized thymoquinone chitosan-polycaprolactone nanoparticles (THQ-CPLNPs).
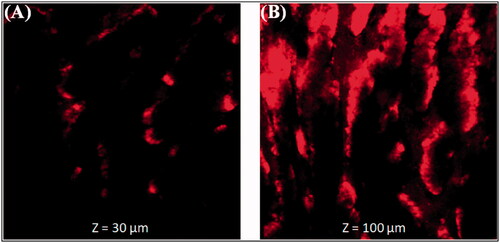
Figure 7. Gastric mucosa irritation study image of (A). Normal saline (B). Thymoquinone suspension (THQ-S) (C). Optimized thymoquinone chitosan-polycaprolactone nanoparticles (THQ-CPLNPs).
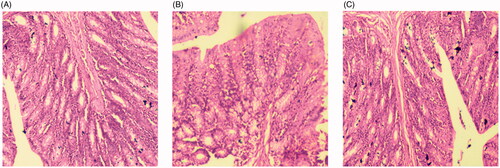
Figure 8. Comparative pharmacokinetic study of optimized thymoquinone chitosan-polycaprolactone nanoparticles (THQ-CPLNPs) and thymoquinone suspension (THQ-S).
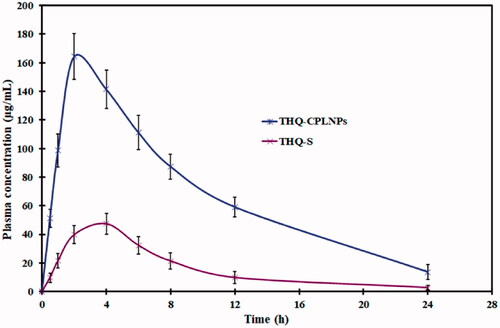
Table 6. Pharmacokinetic parameters of thymoquinone encapsulated chitosan modified polycaprolactone nanoparticles (THQ-CPLNPs) and THQ suspension (THQ-S).
