Figures & data
Table 1. Characteristics of HCC patients.
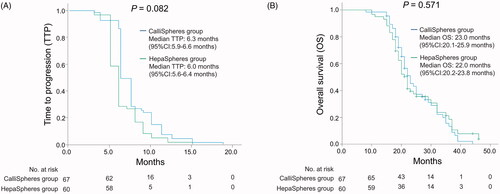
Figure 1. ORR and DCR in CalliSpheres group and HepaSpheres group. The comparison of ORR and DCR at 1 month (A), 3 months (B), and 6 months (C) after treatment between CalliSpheres group and HepaSpheres group. ORR: objective response rate; DCR: disease control rate.
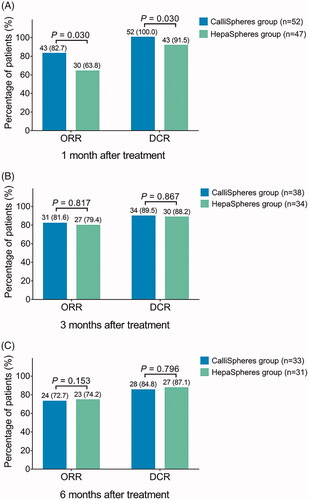
Table 2. Treatment response.
Figure 2. KPS score in CalliSpheres group and HepaSpheres group. The comparison of KPS score at 1 month, 3 months, and 6 months post treatment between CalliSpheres group and HepaSpheres group. KPS: Karnofsky performance status.
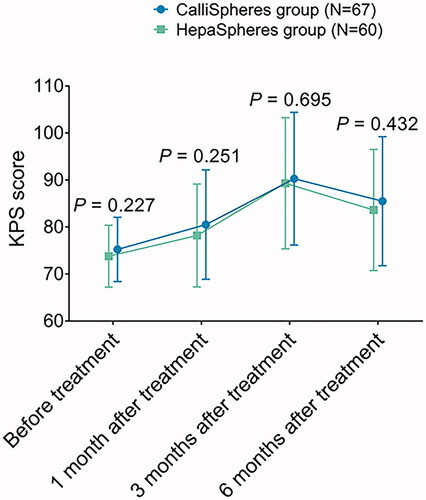
Table 3. Liver function indexes.
Table 4. Adverse events.