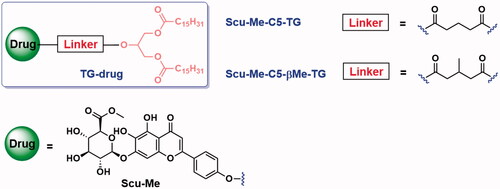
Figures & data
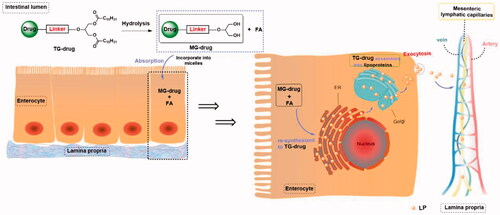
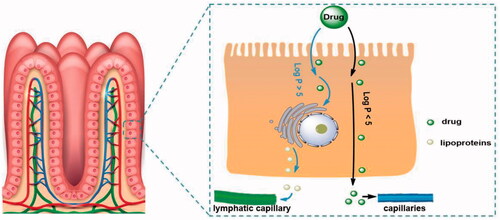
Figure 4. The process by which different drug molecules are absorbed by capillaries and lymphatic capillaries in the intestinal tract.
Scheme 1. Synthetic pathway for prodrugs. (a) Corresponding anhydride, DMAP, DCM, r.t., 24 h, 46.3–50.3%; (b) SOCl2, MeOH, 0 °C, overnight, 82.5%; (c) EDCI, DMAP, DMF, r.t., overnight, 25.6–33.5%.
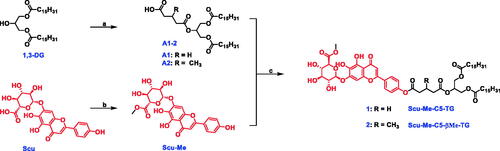
Figure 7. Stability of TG-drug. (A) Chemical stability of TG-drug in simulated gastric fluid; (B) Chemical stability of TG-drug in simulated intestinal fluid, *p < .05. (data are represented as mean ± SD, n ≥ 3).
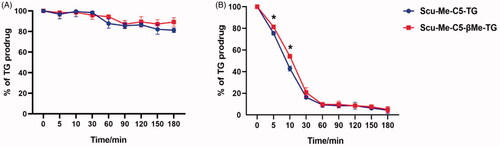
Figure 8. Chemical stability of TG-drug in rat plasma supplying with lipoprotein lipase. (A) The enzymatic hydrolysis rate of TG-drug in rat plasma supplying with lipoprotein lipase; (B) Production of Scu-Me and Scu of the TG-drug upon in vitro incubation with rat plasma supplying with lipoprotein lipase, *p <.05. (data are represented as mean ± SD, n ≥ 3).
Figure 9. Lymphatic absorption of TG-drug. (A) The volume of lymph collected per hour after intragastric administration in female rats; (B) Cumulative lymphatic transport of Scu-Me-C5-TG and Scu-Me-C5-βMe-TG following intragastric administration to anesthetized mesenteric lymphduct-cannulated female rats; (C) Plasma concentrations of free Scu vs. time following oral administration of Scu (25 mg/kg), Scu-Me-C5-TG (62.5 mg/kg equivalent dose of Scu), and Scu-Me-C5-βMe-TG (62.5 mg/kg equivalent dose of Scu) to female rats. *p < .05, **p < .01, ###p < .001. (data are represented as mean ± SD, n ≥ 3).
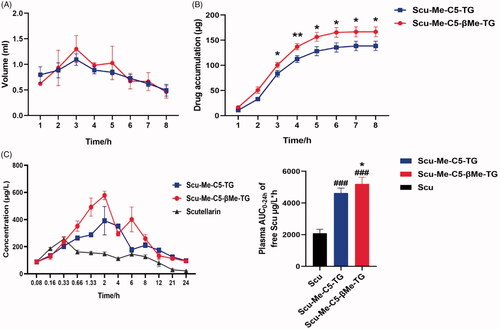
Table 1. Pharmacokinetics parameters for scutellarin, Scu-Me-C5-TG and Scu-Me-C5-βMe-TG after oral administration in female rats (data are represented as mean ± SD, n = 5).