Figures & data
Table 1. Atomic composition of the investigated SP-PC-HA simulated systems.
Table 2. D-optimal design used for optimization of SP-loaded HAECs.
Figure 1. Binding poses of the spironolactone phosphatidyl choline docked complex. (a) In absence of the hyaluronic acid monomer. (b) In combination with the hyaluronic acid monomer (magenta sticks) serving as an anionic formulation additive. Predicted binding interactions are illustrated between the drug molecule; spironolactone (yellow sticks) and of the hyaluronic acid monomer; phosphatidyl choline (green sticks). More favored complex stability was assigned for the spironolactone–phosphatidyl choline in combination with hyaluronic acid. Polar interactions (hydrogen bonding), discussed within the text, are depicted as black dashed lines.
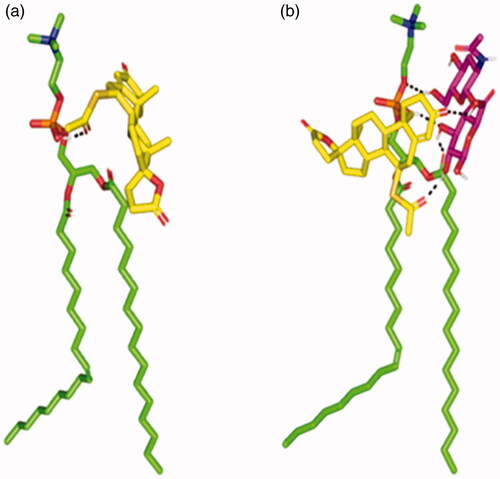
Figure 2. Conformation alterations time evolution of SPH ternary complex throughout the explicit molecular dynamics simulation at different solvation system. The thermodynamic movements and stability of formulation components; spironolactone (yellow sticks), hyaluronic acid monomer (magenta sticks), and phosphatidyl choline (green sticks) were monitored over MD simulation trajectories within (a) 100% chloroform; (b) combined chloroform/alcohol; (c) 100% water solvated systems at captured at different snapshots (1) 0.2 ns; (2) 0.4 ns; (3) 0.6 ns; (4) 0.8 ns; and (5) 1 ns. Polar interactions (hydrogen bonding) are depicted as black dashed lines. (d) Molecular surface 3 D representation of the cone micellar configuration of optimized SPH in chloroform: alcohol (1:1) combination (left panel), as well as the inverted cone micellar configuration of 100% water (right panel). Molecular surface representations were illustrated in colors being previously assigned for the optimized SPH components; yellow, magenta, and green are for spironolactone, hyaluronic acid monomer, and phosphatidyl choline, respectively.
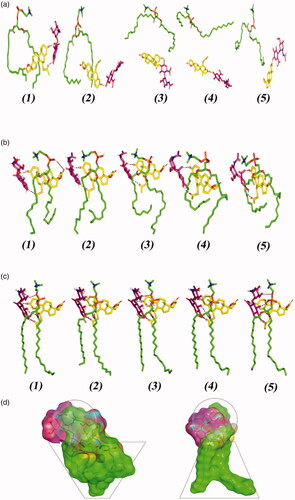
Table 3. Experimental runs, independent variables and measured responses of SP- HAECs.
Table 4. Output data of the D-optimal design analysis of SP-HAECs.
Figure 3. Effect of formulation variables on EE% of SP-HAECs (a–c), PS (d–f), while (g–i) show the effect of significant interactions on PS. Abbreviations: EE%, Entrapment efficiency percentage; PS, Particle size; SP, spironolactone; HAECs, Hyaluronic acid enriched cerosomes.
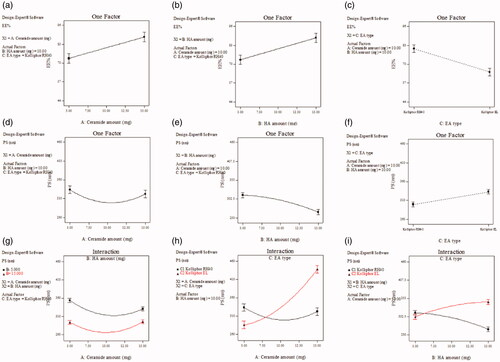
Table 5. Effect of storage on different measurements of OHAEC.
Figure 4. Transmission electron micrographs of SP OHAEC showing mixed vesicular and tubular appearances. Abbreviation: OHAEC, optimal hyaluronic acid enriched cerosomes.
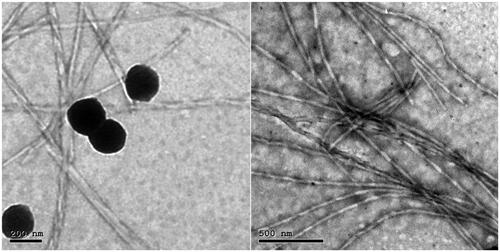
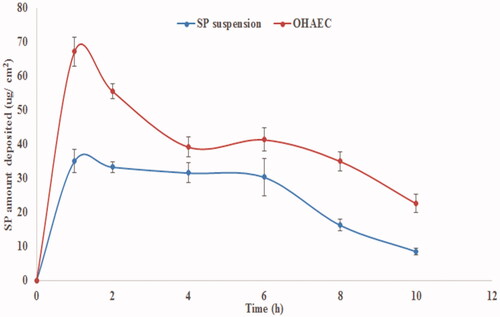
Figure 5. Ex vivoprofile of SP from OHAEC, compared to its aqueous suspension. Abbreviation: SP: spironolactone, OHAEC: optimal hyaluronic acid enriched cerosomes.
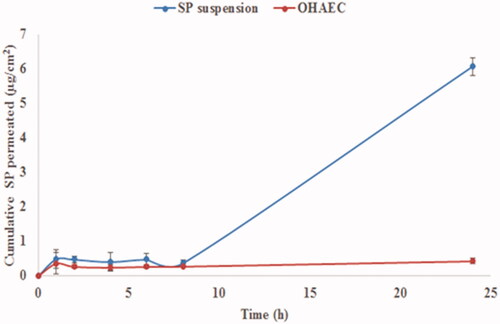
Table 6. Ex vivo permeation and deposition parameters of OHAEC, compared to SP suspension.
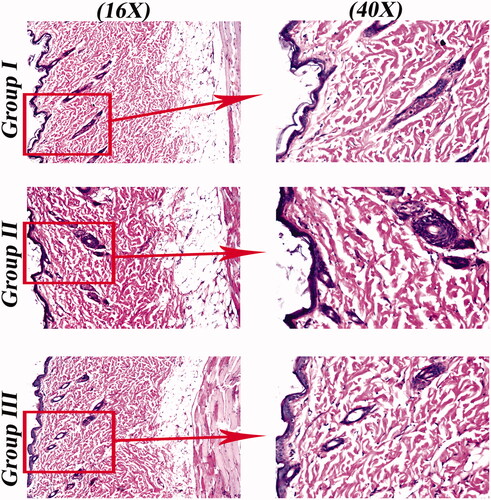
Figure 6. Light microscope photomicrographs showing histopathological sections (hematoxylin and eosin stained) of rat skin normal control (group I), rat skin treated with SP suspension (group II) and rat skin treated with OHAEC (group III) with magnification power of 16X to illustrate all skin layers (Left side) and magnification power of 40X to identify the epidermis and dermis (Right side). Abbreviation: SP: spironolactone, OHAEC: optimal hyaluronic acid enriched cerosomes.