Figures & data
Table 1. Variables and their levels and constraints in D-optimal combined mixture process design.
Table 2. The experimental runs generated by the optimal design and measured responses for the prepared EPL-NCs.
Table 3. Statistical analysis of the experimental data with ANOVA and the predicted and observed results of the optimized EPL-NCs.
Figure 1. 2D contour plots presenting the effects of independent variables on size (A, B) and dissolution efficiency (C, D) of EPL-NCs.
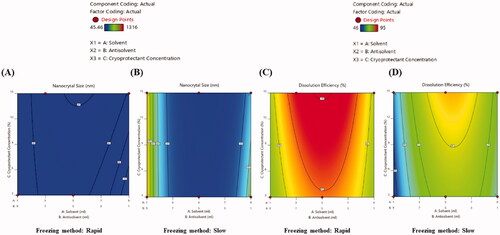
Figure 2. Particle size distribution curve (A) and scanning electron microscopic image (B) of the optimized EPL-NCs.
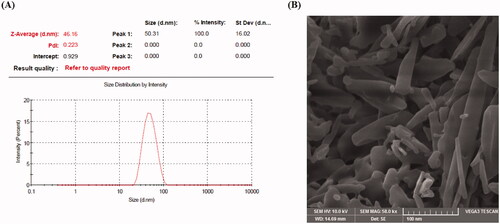
Figure 3. Chemical structure of EPL (A) and FTIR spectra (B) of EPL (a), mannitol (b), physical mixture (c), and EPL-NCs (d).
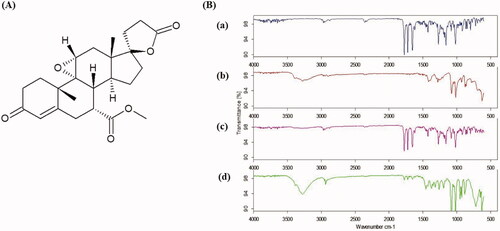
Figure 4. DSC thermograms (A) and PXRD patterns (B) of EPL (a), mannitol (b), physical mixture (c), and EPL-NCs (d).
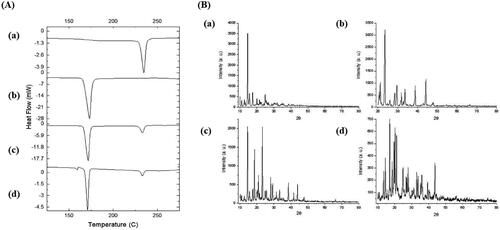
Figure 5. Saturation solubility (A) and dissolution profile (B) of the optimized EPL-NCs. Data are presented as mean ± S.D. (n = 3). *p<.001 vs. EPL powder.
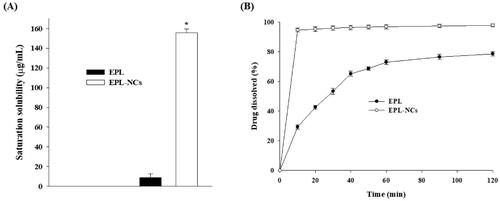
Figure 6. Average plasma level vs. time curve after oral administration of EPL-NCs and EPL powder to rats at a dose equivalent to 10 mg/kg of EPL. Data are expressed as mean ± S.D. (n = 3).
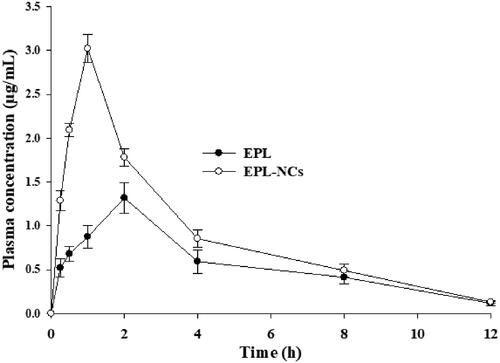
Table 4. Non-compartmental pharmacokinetic parameters after oral administration of EPL-NCs and EPL powder to rats at a dose equivalent to 10 mg/kg of EPL.
Figure 7. Organ-to-body weight index (A) and histological microphotographs of liver, kidney and heart tissues of mice (B) at day 14 after treatment with EPL-NCs. In histological micrographs, (1) hepatocytes, (2) hepatic artery, (3) sinusoids, (4) central vein, (5) bile duct, (6) bowman capsule, (7) glomerulus, (8) renal tubule, (9) proximal tubule, (10) distal tubule, and (11) myocardial fibers. Organ-to-body weight indices are presented as mean ± S.D. (n = 6).
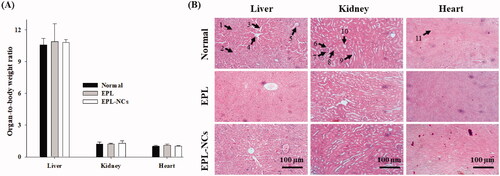
Figure 8. Blood hemolysis by the optimized EPL-NCs indicated with real-time photographs of RBCs samples (A) and quantified with spectroscopic analysis of hemoglobin at 541 nm (B). The RBCs were mixed with Triton X-100 solution (positive control), isotonic PBS (negative control), 20, 40, 60, 80, and 100 µg/mL of EPL (D-1 to D-5) and 20, 40, 60, 80, and 100 µg/mL of EPL-NCs (F-1 to F-5), respectively. For the quantification of hemolysis (%), data are presented as mean ± S.D. (n = 3).
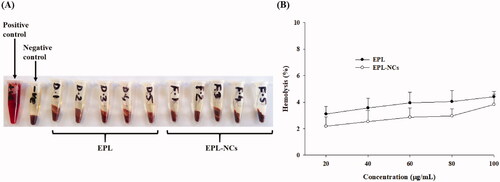
Figure 9. DSC thermograms (A) and PXRD patterns (B) of EPL-NCs at day 0 (a) and after 90 days (b) of storage stability period.
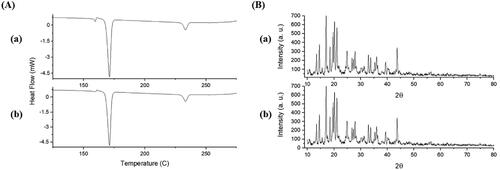
Table 5. Storage stability of optimized EPL-NCs at 40 °C for 90 days.
