Figures & data
Figure 1 Current difference-concentration curves obtained for the electrooxidation of H2O2 at Pt, PVF+ClO4− modified and enzyme electrodes: ▪: Pt, ▴: PVF+ClO4− modified, ♦: creatine enzyme electrodes (0.05M pH 7.5 phosphate buffer, 25°C).
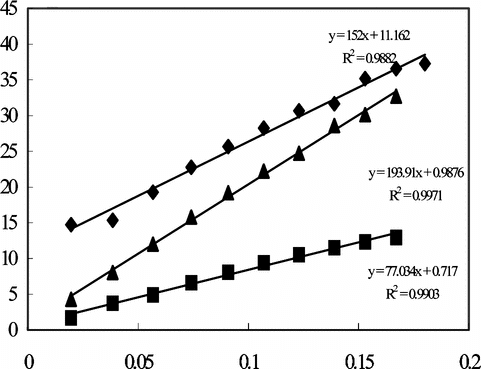
Figure 2 Creatine response of the enzyme electrodes, İmmobilization method; ♦: Adsorption ▪: Immersing into GA ▴: Crosslinking with GA-BSA (0.05 M pH 7.5 phosphate buffer, 25°C).
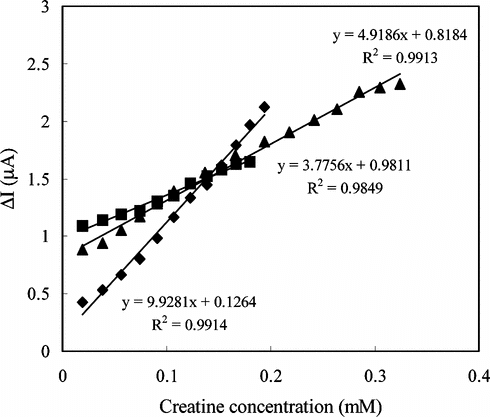
Figure 3 The reproducibility of the enzyme electrode (Immobilization method: adsorption; 0.05 M pH 7.5 phosphate buffer, 25°C).
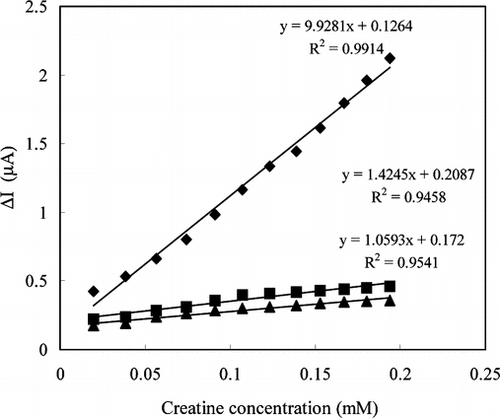
Figure 4 The reproducibility of the enzyme electrode prepared by immersing into GA (0.05 M pH 7.5 phosphate buffer, 25°C).
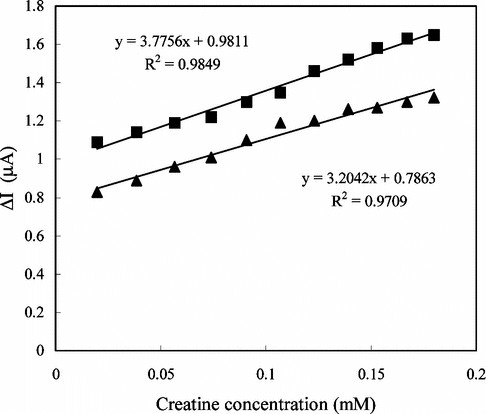
Figure 5 The effect of the solution (1.4 × 10−5 M creatine) pH on the response of the electrode in 0.05 M phosphate buffer solution at 25°C.
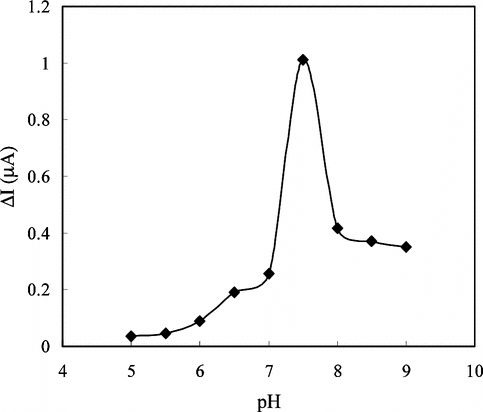
Figure 6 The effect of temperature on the response of enzyme electrode against creatine (0.05 M pH 7.5 phosphate buffer).
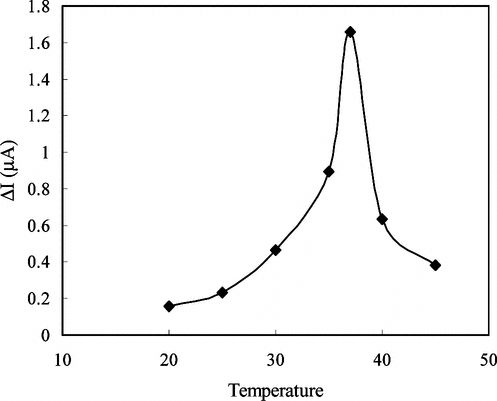
Figure 7 The effect of the phosphate concentration on the response of the enzyme electrode against creatine (pH 7.5 phosphate buffer, 25°C).
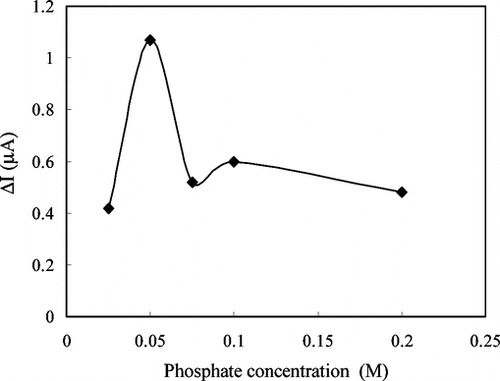
Figure 8 The effect of enzyme ratio on the response of enzyme electrode against creatine (0.05 M pH 7.5 phosphate buffer, 25°C).
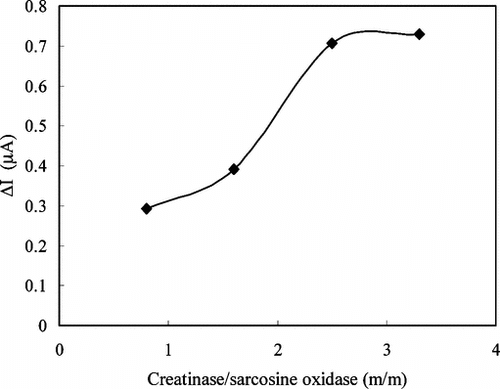
Figure 9 The reproducibility of the electrode prepared by crosslinking with GA-BSA system (0.05 M pH 7.5 phosphate buffer, 25°C).
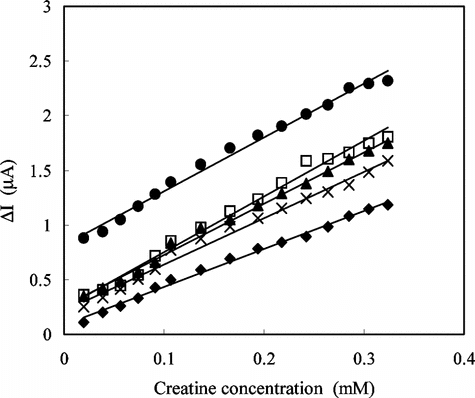
Figure 10 Storage stabilization of the enzyme electrode prepared by crosslinking with GA-BSA system (0.05 M pH 7.5 phosphate buffer, 4°C).
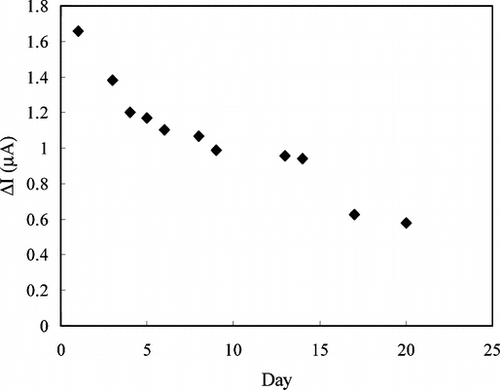
Table 1 The properties and the optimum working conditions of the enzyme electrode