Figures & data
Figure 1 Four chains of hemoglobin are shown in the form of ribbon. Light gray chains correspond to α type and dark gray chains correspond to β. All the eight sites are shown with cys residues in CPK model. The cys atoms color coded with white correspond to the sulfur atoms.
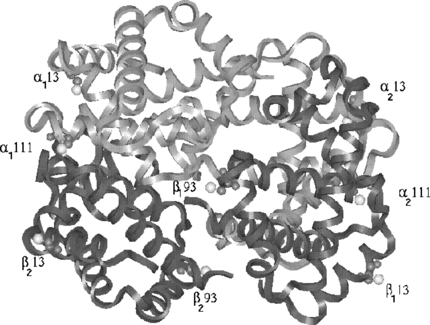
Figure 2 The distances between the sites are shown to show the adequate coverage of the protein surface. The shortest distance is 13 Å and widest distance is 56.8 Å.
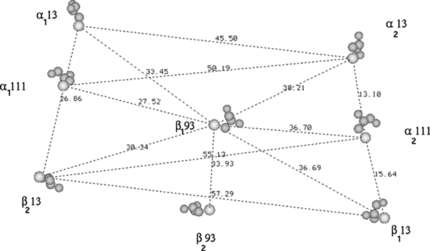
Figure 3 a) Structure of the poly(ethylene glycol)-based mono functional maleimide reagents. The number n gives the number of PEG molecules in the various chains in our study; b) The maleidophenyl linkage between cys residue and the PEG chains.
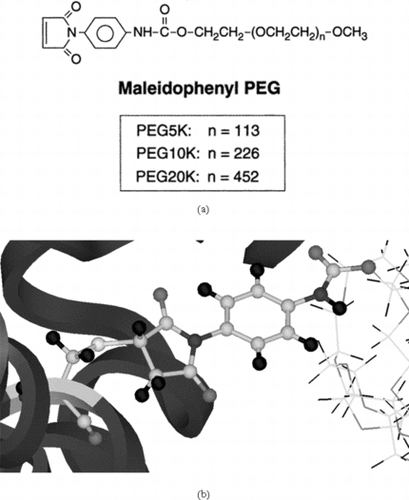
Figure 4 The three-dimensional models of the 5K, 10K and 20K PEG chains involved in our analysis. The connecting maleimide segment between the PEG and protein is shown in CPK type.
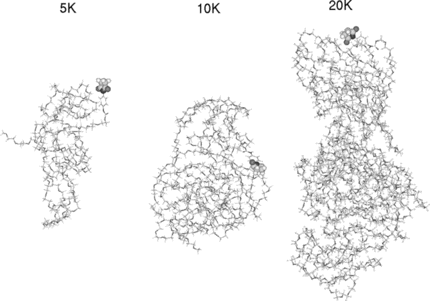
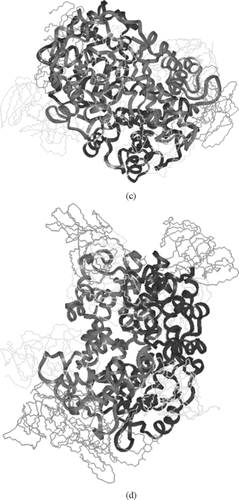
Figure 5 a)Symmetric linkage of 2 5k PEG chains with Hemoglobin at β93; b) Symmetric linkage of 4 5k PEG chains with Hemoglobin at β93 & α111; c) Symmetric linkage of 6 5k PEG chains with Hemoglobin at β93, α111 & α13; d) Symmetric linkage of 8 5k PEG chains with Hemoglobin at β93, α111 α13 & β13 (hydrogen are omitted in c & d for clarity).
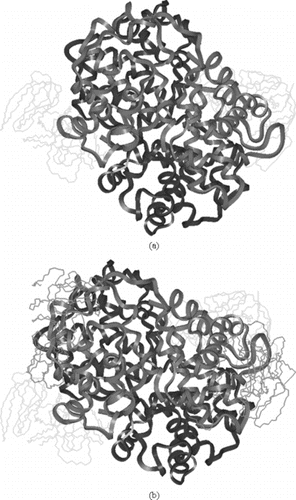
Figure 6 The accessible surface area for the water molecule of 1.4 Å radius at various stages of PEGylation of 5 K mass (2, 4, 6, & 8) are shown. The zero corresponds to the crystal structure.
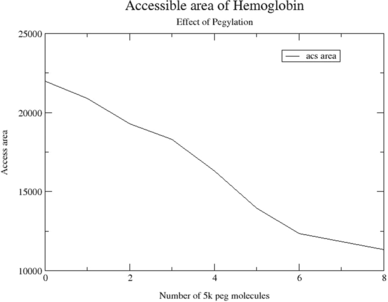
Figure 7 a) Symmetric linkage of 4 10k PEG chains with hemoglobin at β93 & α111; b) Symmetric linkage of 2 20k PEG chains with hemoglobin at β93 (hydrogen are omitted). In , and have the attachment of same mass.
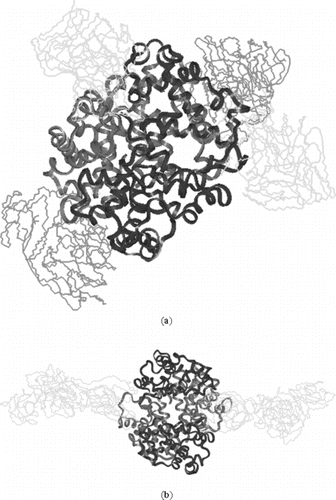
Table 1. Area & volume of modified hemoglobins