Figures & data
Table 1 The membrane compositions of ammonium-ion selective electrodes
Figure 1 Calibration curve for ammonium ion. Electrode I: with 3% nonactin prepared by using PVC membrane containing palmitic acid. Electrode II: with 3% nonactin prepared by using carboxylated PVC membrane.
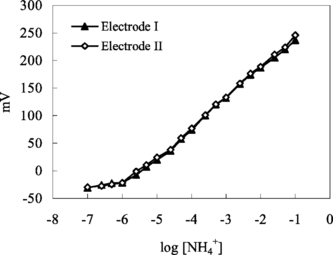
Figure 2 Calibration curve for ammonium sensor. Electrode IV: with 4% nonactin prepared by using PVC membrane containing palmitic acid. Electrode V: with 4% nonactin prepared by using carboxylated PVC membrane.
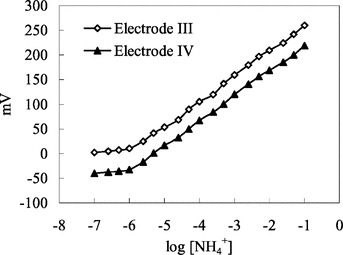
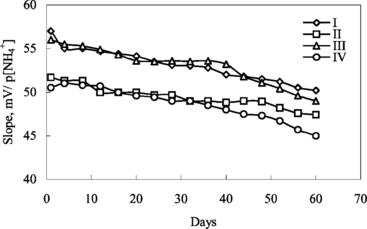
Table 2 Slopes and linear working ranges of the ammonium electrodes at 20 ± 1°C
Figure 3 The effect of buffer concentration on ammonium-selective sensors. I and II: electrodes with 3% and 4% nonactin prepared by using PVC containing palmitic acid; III and IV: electrodes with 3% and 4% nonactin prepared by using carboxylated PVC, respectively.
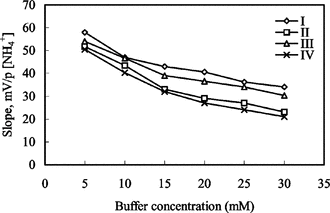
Figure 4 The effect of pH on ammonium sensors. I and II: electrodes with 3% and 4% nonactin prepared by using PVC containing palmitic acid; III and IV: electrodes with 3% and 4% nonactin prepared by using carboxylated PVC, respectively.
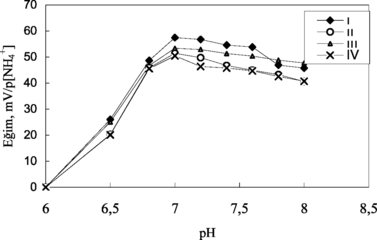
Figure 5 The effect of temperature on ammonium-selective sensors. I and II: with 3% and 4% nonactin prepared by using PVC containing palmitic acid; III and IV: with 3% and 4% nonactinprepared by using carboxylated PVC, respectively.
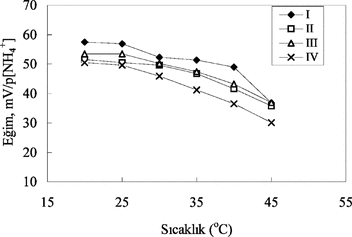
Table 3 Selectivity coefficients of ammonium electrodes