Figures & data
Table 1. Pseudo-ACE activity of Hbs toward N-[3-(2-furyl)acryloyl]-L-phenylalanylglycylglycine (FAPGG), the ACE synthetic substrate that mimics Ang-I
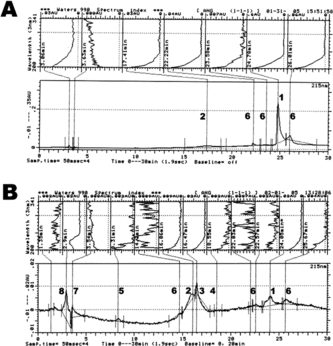
Figure 1 C-18 reversed phase chromatographic (bottom) and spectral (top) profiles of different angiotensin metabolites produced following the reaction of 77 µM Ang-I with: saline-control (A), 77 µM of hydrogen peroxide (1 : 1 molar ratio with respect to Ang-I) (B), 7.7 µM of ferrous-Hb (1:10 molar ratio with respect to Ang-I) (C), and 7.7 µM of Hb reacted with 77 µM of hydrogen peroxide (1:2.5 molar ratio in respect to heme; ferryl-Hb) (D). Chromatographic Fraction Identifiers: 1 = Angiotensin I; 2 = Angiotensin II; 3 = Angiotensin III; 4 = Angiotensin IV; 5 = Angiotensin (1–7) Fragment; 6 = Other angiotensin metabolites; 7 = Heme byproducts; 8 = Oxidative products of the reaction Between Hb and hydrogen peroxide. The reaction conditions and C-18 separation method are described in the Materials and Methods section. The integrator analysis (area %) of the obtained fractions is presented in the Results section.
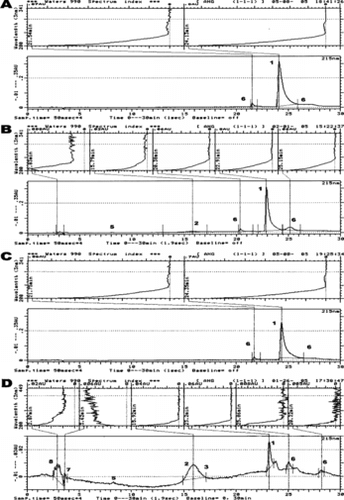
Table 2. Effects of Hbs and/or hydrogen peroxide on the conversion rate of Ang-I into Ang-II, determined on the basis of the immunological identification of Ang II with the ELISA method
Figure 2 C-18 reversed phase chromatographic (bottom) and spectral (top) profile of different angiotensin metabolites produced following the reaction of 77 µM Ang-I with: 216 µM hydrogen peroxide (1:2.8 molar ratio with respect to Ang-I) (A) and 7.7 µM Hb reacted with 216 µM of hydrogen peroxide (1:7 molar ratio with respect to heme; ferryl Hb) (B). Chromatographic identifiers are the same as in .