Figures & data
Figure 1 Detection of SARS coronavirus M, S, N protein in COS-1, Huh-7, HepG2 by Western blot analysis. The results are representative of at least three independent experiments. M, N protein is detected in 15% SDS-PAGE gel, S protein is detected in 10% SDS-PAGE gel. In mock (pCDNA 3.1-) transfected cells, no land can be detected in the corresponding site.
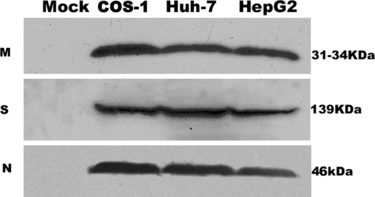
Figure 2 Annexin V detection of apoptosis induced by M, S and N protein of SARS coronavirus in COS-1, HepG2, Huh-7 cells. Cells were analyzed by flow cytometry with FITC-labeled annexin V and propidium iodide under 24 hours serum withdrawal. (A) Panels are transfected by mock M, S and N of SARS coronavirus from left to right in COS-1, HepG2, Huh-7 cells. In each panel (bottom left) viable cells (PI −, annexin-V/FITC −). (Bottom right) Early apoptotic cells (PI −, annexin-V/FITC +). (Top right) Non-viable, late apoptotic/necrotic cells (PI +, annexin-V/FITC +). (Top left) Necrotic cells(PI +). Numbers in the quadrants indicate the proportions of cells in the corresponding areas. Data from one representative experiment are presented. Experiments were performed in triplicate, with similar results. (B) Data are means±S.D. of triplicates from representative experiments. In COS-1 cells, apoptosis induced by SARS coronavirus N protein is 28.75%±1.93, **p < 0.01 vs. mock transfected.
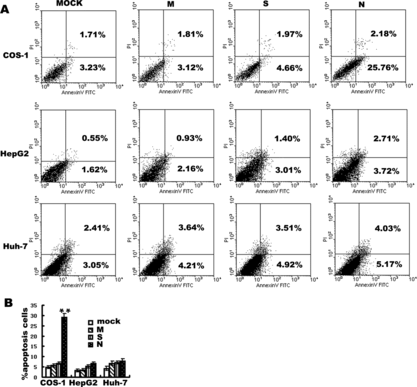
Figure 3 Electron microscopy (EM) of SARS N transfected three cell lines. a1: Mock transfected COS-1 cell with a normal nucleus, well-preserved cytoplasm, containing well-preserved mitochondria. a2: SARS coronavirus N transfected COS-1 cell shows large dark mass of electron dense material in the nucleus, which clumped into a uniform sphere with a degenerated cytoplasm, comparable to that of mock transfected cells. b1: mock transfected HepG2. b2: N transfected HepG2.c1: mock transfected Huh-7 .c2:N transfected Huh-7. There is no obvious change in N transfected cells HepG2 and Huh-7 cells. Magnification: a1, b1, b2, c1,c2 (5000 ×), a2 (6700 ×).
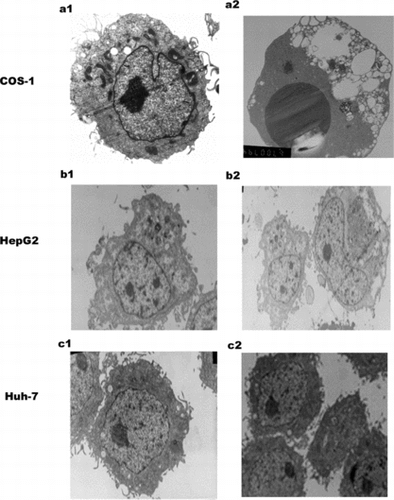
Figure 4 Western blotting analysis of activation of caspase-3 and cleavage of PARP in COS-1. Cells were mock or SARS coronavirus N transfected without or with z-VAD-fmk 100 uM. Caspase-3 and PARP were analyzed by separation of total proteins on 18% and 10% SDS-polyacrylamide gels, respectively, for three times.
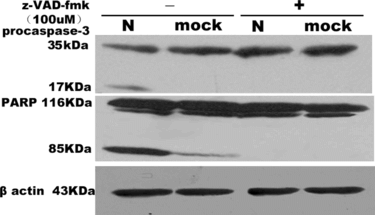
Figure 5 Flow cytometry analysis of the effect of SARS coronavirus N protein on production of ROS (A) and decrease of ΔΨm (B) in COS-1 cells. Transfected cells were subjected to redox-sensitive fluorescent dye DCF-DA (A) and ΔΨm -sensitive fluorescent dye DiOC6 (B). Fluorescence related to intracellular ROS production and loss of ΔΨm were both measured in the fluorescence signal in the FL1 channel. Transfected cells were subjected to 12 hours, 18 hours, 24 hours serum withdrawal. The mean fluorescence intensity of cells with reduced DiOC6 or increased DCF staining is shown. In the left three panels of A and B, every experiment performed in triplicate with similar results and data of the panels are presented from a representative experiment. The right panels are the statistic results, which are means±S.D. from at least three experiments, *represented P < 0.05, **represented P < 0.01 compared to mock transfected cells. A.U. means Arbitrary Units.
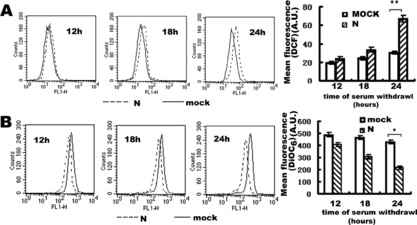
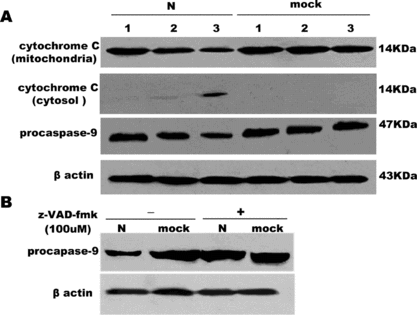
Figure 6 The release of cytochrome C into cytosol and activation of caspase-9 in COS-1 cells by 15% SDA-PAGE. (A) The decrease of cytochrome C in mitochondrial, the release of cytochrome C in the cytosol, activation of procaspase-9 were examined in a time-dependent course. Lane 1, 2, 3 represents serum withdrawal for 12, 18, 24 hours, respectively, in SARS coronavirus N or mock-transfected COS-1 cells. (B) Effect of z-VAD-fmk on the activation of caspase-9 induced by N or mock-transfected COS-1 in the absence or presence of z-VAD-fmk (100 uM) under 24 hours' serum withdrawal. β Actin was loaded as loading controls. Pictures are representative of one of three similar results.