Figures & data
Figure 1. Construction of plasmids pGEX and pET-Duet 1 containing Arenicola marina B2 globin cDNA for the production in BL21 E. coli. Map of pGEX-B2 (a) and pET DUET-1 B2 (b) plasmids.
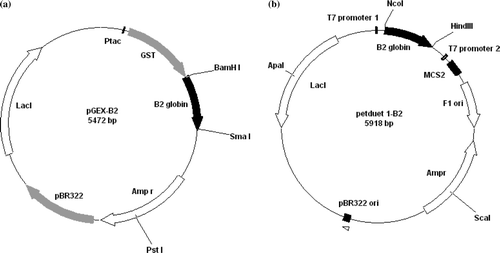
Figure 2. Expression and purification of recombinant B2-GST globin. (a) Analysis of the purified B2-GST globin by SDS/PAGE on a gradient 4–20% polyacrilamide gel after Coomassie blue staining. Lane 1, molecular weight markers (kDa shown on the left); lane 2, total fraction of soluble proteins from cells containing the pGEX-B2 plasmid; lane 3, soluble B2-GST after purification and elution from the bound glutathione; lane 4, cleavage B2-GST by factor Xa; lane 5, purification by gel filtration Superdex 75. (b) Elution profile of the B2-GST globin on a superdex 75 10/300GL column after cleavage by factor Xa. The elution was followed by the absorbance at 280 nm (solid line) and 414 nm (dashed line).
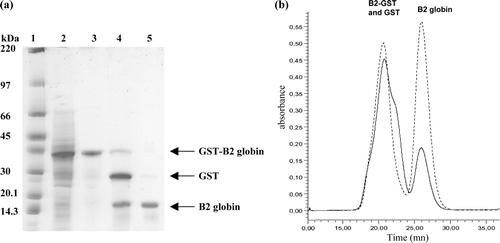
Table 1. Comparison of the recombinant globins yield
Figure 3. Expression and purification of recombinant RecB2 globin. (a) Analysis of the purified RecB2 globin by SDS/PAGE on a gradient 4–20% polyacrilamide gel after Coomassie blue staining (1–2 µg proteins/lane). Lane 1, molecular weight markers (kDa shown on the left); lane 2, total fraction of soluble proteins from cells containing the pET-Duet-B2 plasmid; lane 3, supernatant fraction obtained after ammonium sulfate fractionation 60%; lane 4, first purification by DEAE-Sepharose; lane 5, second purification by gel filtration Superdex 75. (b) Elution profile of the RecB2 globin on a superdex 75 10/300GL column after purification on DEAE Sepharose. The elution was followed by the absorbance at 280 nm (solid line) and 414 nm (dashed line).
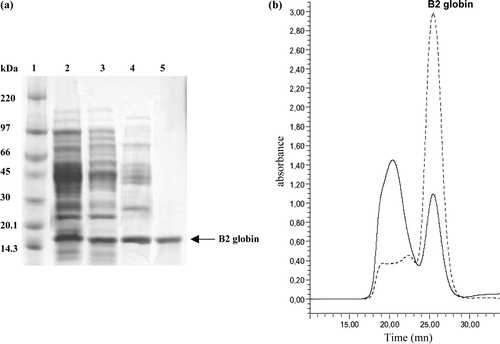
Figure 4. ESI-MS of overexpressed B2 globin. (a) Smoothed, multiply charged ESI mass spectrum. (b) Corresponding deconvoluted spectrum. (c) List of the peptide sequences identified using the Mascot search engine in Arenicola marina globin B2 (Q53I64) (from the LC-MS/MS analysis of the digested 1D-SDS PAGE band). (d) Product ion spectra of the doubly charged precursor ion at 706.25 m/z (the spectrum is deconvoluted into (M + H)+ species). The proposed amino acid sequence for the N-terminus peptide (including a modified Met having a side chain mass of 169 Da) and the expected yn (C-terminus ions) and bn (N-terminus ions) fragment-ions are represented on the spectrum.
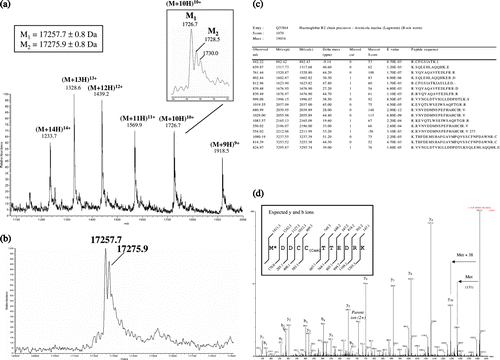
Figure 5. UV/Vis Spectra of RecB2 (a) and B2-GST (b). Absorption spectra were measured in saline buffer buffer (see Materials and Methods) at 25°C for ferric hexacoordinated form (long-dashed line), deoxy ferrous pentacoordinated form (short-dashed line) and oxy form (solid line).
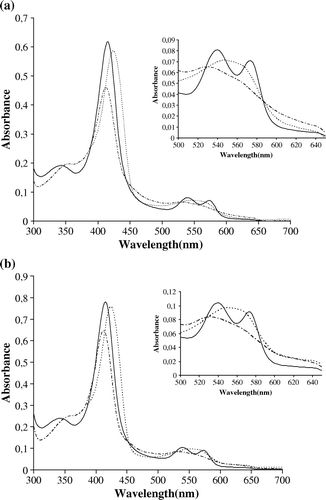
Figure 6. Auto-oxidation kinetics of RecB2 (♦) and B2-GST (•), compared with those of Hba (▴) and HBL-Hb (▪). The transition of the ferrous oxy to ferric hexacoordinated form is shown on the graph (b). Experimental conditions were 100 mM potassium phosphate buffer pH 7.0, with 1 mM EDTA.
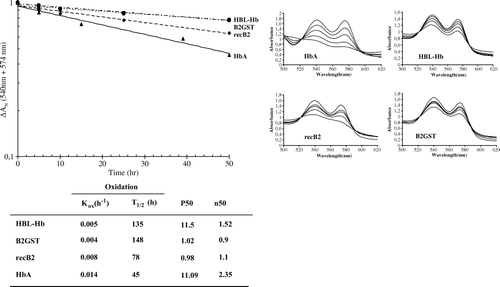
Table 2. Functional properties