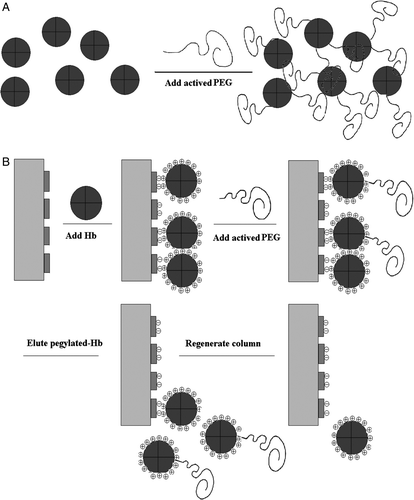
Figures & data
Figure 1. Simplified scheme of the reaction between succinimidyl carbonate mPEG (SC-mPEG) and hemoglobin.
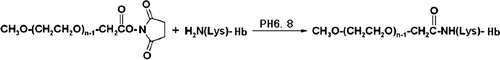
Figure 2. HPSEC analysis of pegylated hemoglobin in liquid phase. Samples were loaded on a Superdex 200 gel filtration column with 50 mM sodium phosphate containing 0.15 M sodium chloride (pH 7.4) as the mobile phase at a flow rate of 0.5 ml/min. A was the nature hemoglobin, and B to D were the results of pegylated-Hb with PEG5kDa, PEG10kDa, and PEG20kDa, respectively.
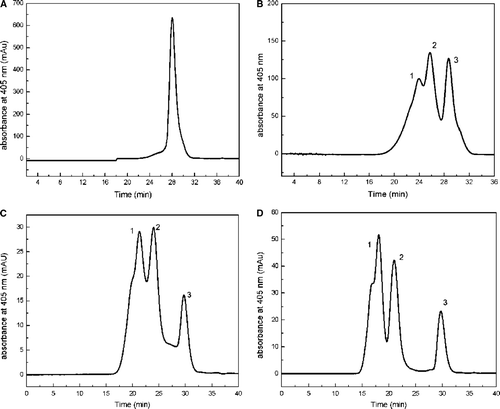
Figure 3. Elution process of the pegylated hemoglobin using ion-exchange chromatography on CM Sepharose Fast Flow. The column was equilibrated with 10 mM PBS at pH6.8, and the column was eluted with 1M NaCl in 10 mM PBS at pH6.8. The flow rate was 1.0 ml min/ml and the elution profiles were monitored by absorbance at 280 nm and 405nm.
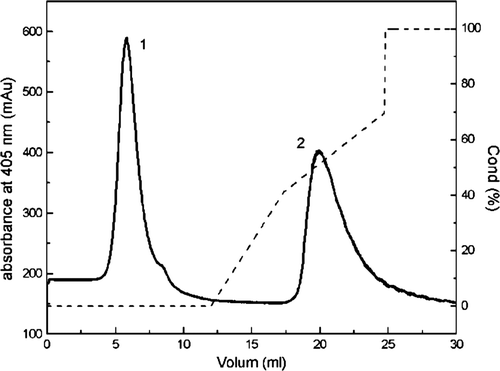
Figure 4. HPSEC-MALLS chromatograms analysis of PEG5kDa-modified hemoglobin by solid adsorption method. The samples were loaded on a Superdex 200 gel filtration column with 50 mM sodium phosphate containing 0.15 M sodium chloride (pH 7.4) as the mobile phase at a flow rate of 0.5 ml/min. A was the result of the first peak (F1) by HPSEC; B was the result of the first peak (F1) by HPSEC-MALLS; C was the result of the second peak (F2) by HPSEC; D was the result of the second peak (F2) by HPSEC-MALLS.
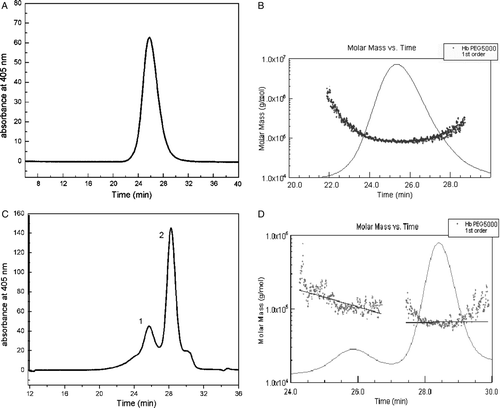
Figure 5. HPSEC-MALLS chromatograms analysis of PEG10kDa-modified hemoglobin by solid adsorption method. The samples were loaded on a Superdex 200 gel filtration column with 50 mM sodium phosphate containing 0.15 M sodium chloride (pH 7.4) as the mobile phase at a flow rate of 0.5 ml/min. A was the result of the first peak (F1) by HPSEC; B was the result of the first peak (F1) by HPSEC-MALLS.
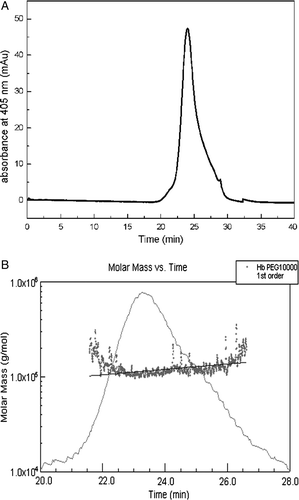
Figure 6. HPSEC-MALLS chromatograms analysis of PEG20kDa-modified hemoglobin by solid adsorption method. The samples were loaded on a Superdex 200 gel filtration column with 50 mM sodium phosphate containing 0.15 M sodium chloride (pH 7.4) as the mobile phase at a flow rate of 0.5 ml/min. A was the result of the first peak (F1) by HPSEC; B was the result of the first peak (F1) by HPSEC-MALLS.
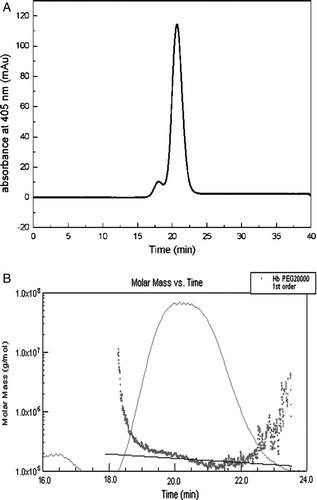
Figure 7. SDS-polyacrylamide gel electrophoresis of Pegylated-Hb with PEG5kDa. The gel concentration is 13.5% and was stained by silver. Lane 1, natural hemoglobin; Lane 2, the result of intramolecularly cross-linked hemoglobin; Lane 3, standard protein markers; Lane 4, the result of modified-Hb by liquid phase; Lane 5, the result of the first elution peak by solid phase; Lane 6, the result of elution peak by solid phase.
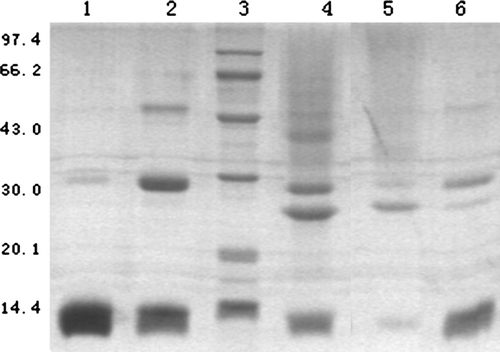
Figure 8. SDS-polyacrylamide gel electrophoresis of Pegylated-Hb with PEG10kDa and PEG20kDa. The gel concentration is 13.5% and was stained by silver. Lane 1, natural hemoglobin; Lane 2, the result of intramolecularly cross-linked hemoglobin; Lane 3, the result of PEG10kDa-modified hemoglobin by liquid phase; Lane 4, the result of PEG20kDa-modified hemoglobin by liquid phase; Lane 5, standard protein markers; Lane 6, the result of the first elution peak of PEG10kDa-modified hemoglobin by solid phase; Lane 7, the result of elution peak of PEG10kDa-modified hemoglobin by solid phase; Lane 8, the result of the first elution peak of PEG20kDa-modified hemoglobin by solid phase; Lane 9, the result of elution peak of PEG20kDa-modified hemoglobin by solid phase.
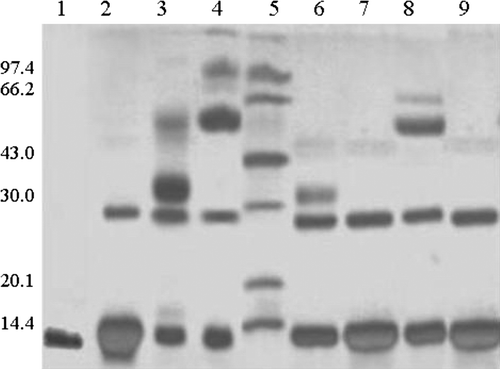
Figure 9. O2 equilibrium curves of pegylated hemoglobin. A was the result of natural hemoglobin; B was the result of PEG5kDa-modified hemoglobin by solid phase; C was the result of PEG5kDa -modified hemoglobin by liquid phase.
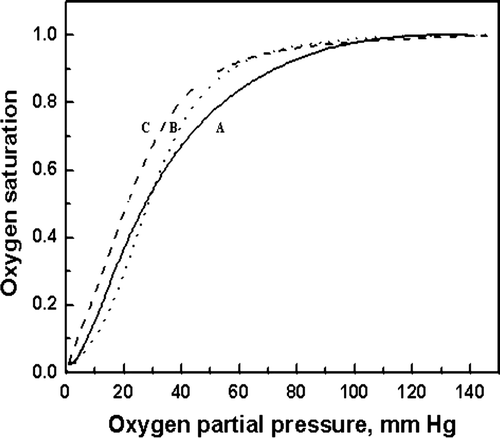
Table 1. The biological activity of the pegylated hemoglobin and the yield of mono-pegylated-Hb