Figures & data
Figure 1. The percent number of Jurkat cells alive, cultured in the presence of polyelectrolytes adsorbed on modified polypropylene support: poly-L-lysine (PLL) layer, bilayer of poly-L-lysine and poly-ethylenimine (PLL/PEI), or poly-L-lysine and poly-styrenosulfonate (PLL/PSS).
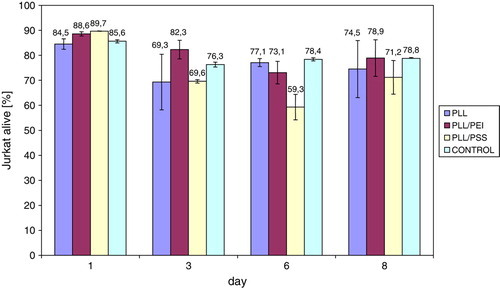
Figure 2. The percent number of Jurkat cells alive, after 5-day culture in the presence of polyelectrolytes adsorbed on modified polypropylene support: poly-allylamine (PAH) layer, or bilayer of poly-allylamine and poly-ethylenimine (PAH/PEI), or poly-allylamine and poly-styrenosulfonate (PAH/PSS).
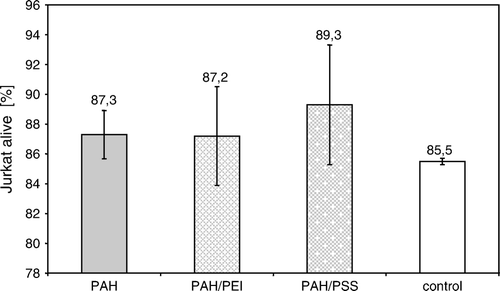
Figure 3. The mean yield of nanocalsules of 5 milions HL-60 cells. PLL/PEI 1bl – cells covered with poly-L-lysine and polyethylenimine bilayer; PLL/PEI 3bl – cells covered three times with poly-L-lysine and polyethylenimine bilayer (cumulatively 6 layers); PLL/PSS 1bl–cells covered with poly-L-lysine and poly-styrenosulfonate bilayer; PLL/PSS 3 bl-cells covered three times with poly-L-lysine and poly-styrenosulfonate (cumulatively 6 layers); bl-bilayer.
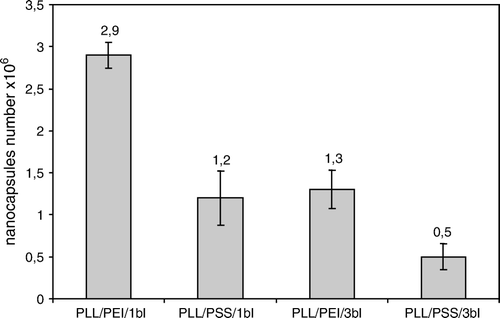
Figure 4. The mean formazan production by nanoencapsulated and non-encapsulated (control) HL-60 cells. PLL/PEI 1bl – cells covered with poly-L-lysine and poly-ethylenimine; PLL/PEI 3bl – cells covered three times with poly-L-lysine and poly-ethylenimine bilayer (cumulatively 6 layers); PLL/PSS 1bl – cells covered with poly-L-lysine and poly-styrenosulfonate; PLL/PSS 3 bl-cells covered three times with poly-L-lysine and poly-styrenosulfonate (cumulatively 6 layers).
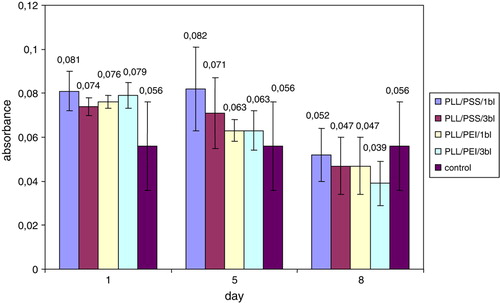
Figure 5. The mean formazan production by non-encapsulated (control) and nanoencapsulated rat islets of Langerhans during 48-hour culture. PAH – islets covered with poly-allylamine layer; PAH/PSS – islets covered with bilayer of poly-allylamine hydrochloride and poly-styrenosulfonate.
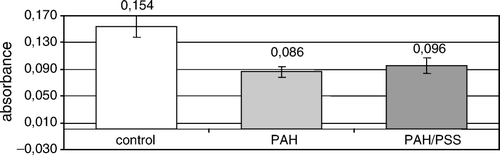
Figure 6. The mean formazan production by non-encapsulated (control) and nanoencapsulated rat islets of Langerhans during 8-day culture. PLL/PEI – islets covered three times with poly-L-lysine and poly-ethylenimine bilayer (cumulatively 6 layers).
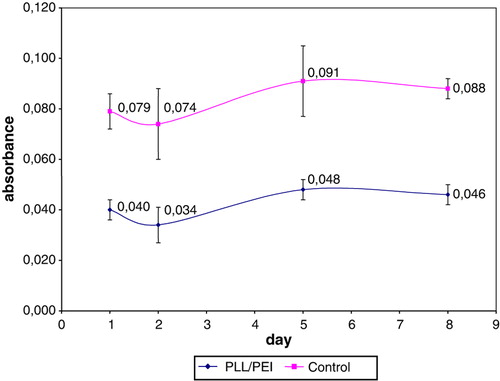
Figure 7. Pancreatic rat islet coated with polyelectrolytes bilayer. SEM image (TM 1000, Hitachi, Japan), ×4000.
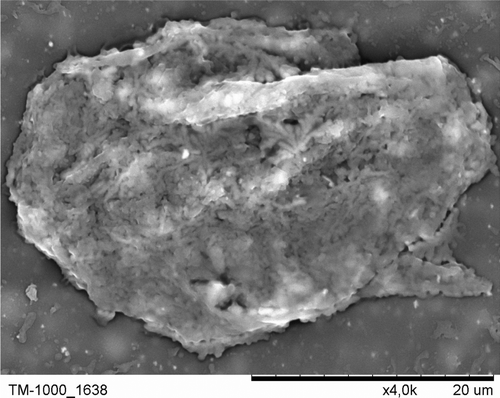
Figure 8. Light microscopic image of PE-coated rat pancreatic islets of Langerhans stained with dithizone.
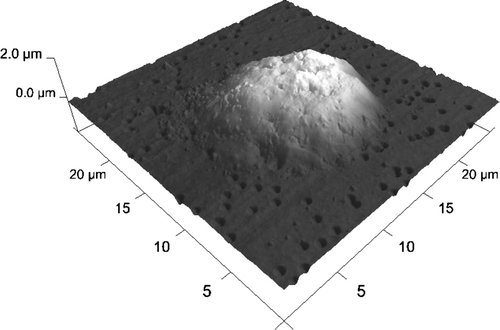
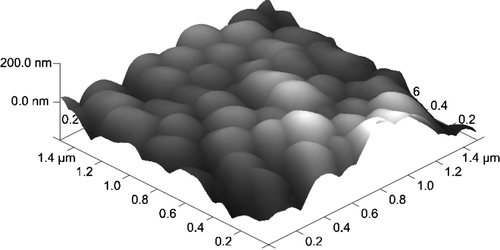
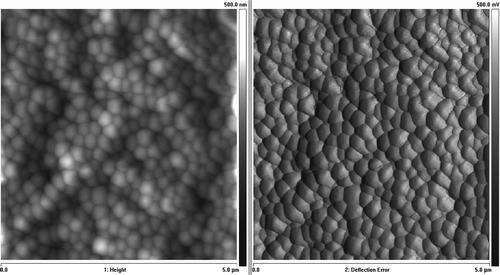
Figure 9. HL-60 cell coated with triple bilayer of poly-L-lysine and poly-ethylenimine, deposited on filtration membrane. AFM image of surface (Nanoscope V, Veeco Instruments Inc., USA).