Figures & data
Table 1. Accuracy, precision, and extraction recovery data for caffeine citrate at concentrations of 1, 15, and 30 µg/mL.
Figure 1. Stacked chromatograms of blank and spiked saliva samples (15 µg/mL) and calibration standard (15 µg/mL) at 273 nm, with caffeine peak eluting at 6.35 min. Graphs in the dotted box show caffeine spectra and representative peak purity of the samples.
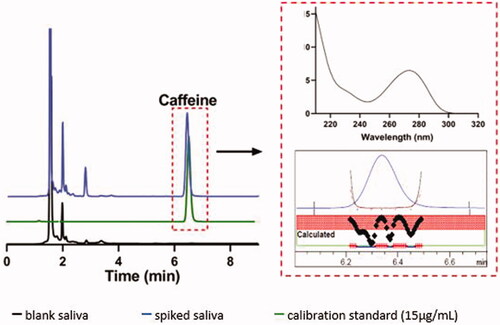
Table 2. Stability of caffeine in saliva under refrigeration (2–8 °C), at room temperature (20–25 °C), and frozen (−20 °C).
Table 3. Stability evaluation of extracted caffeine samples from saliva kept in auto sampler for 72 hr and for three freeze-and-thaw cycles (from −20 °C to room temperature) for n = 3 samples each.
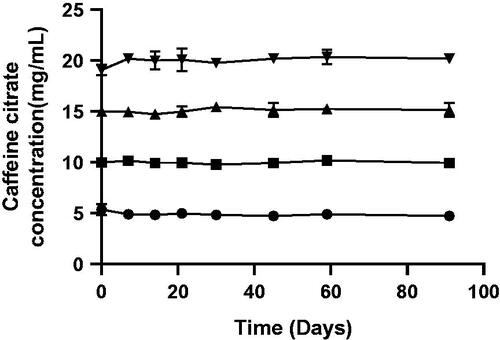
Table 4. pH of caffeine citrate samples [mean (SD); n = 3] stored at room temperature for up to 3 months.