Figures & data
FIGURE 1. Fluid turnover and lymphatic drainage from the pleural space. In the normal pleural space (shown here), as in other interstitial spaces of the body, liquid slowly filters from systemic capillaries and is absorbed via lymphatics (solid arrows). In the pleural space, the capillary filtrate from systemic capillaries moves across a permeable pleural membrane toward the lower pressure pleural space and is absorbed via the parietal pleural lymphatics. From there, liquid moves via lymphatic propulsion to the central veins. When interstitial edema forms in the adjacent lung, some of that excess liquid moves across the visceral pleura into the pleural space. Asbestos fibers may follow similar routes from the lung to the pleura and are thought to lodge in the parietal pleura preferentially at sites of lymphatic drainage.
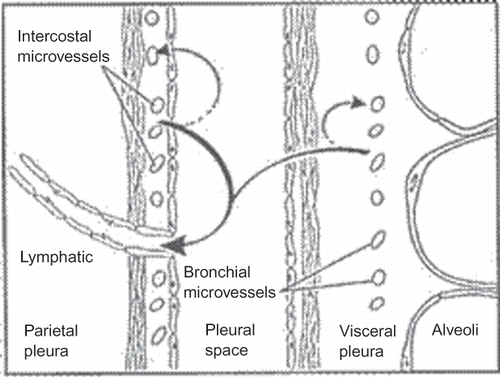
TABLE 1. Asbestos Fiber Content in Lung Tissue of an Urban Population
TABLE 2. Lung Fiber Burdens in Malignant Mesothelioma Patients
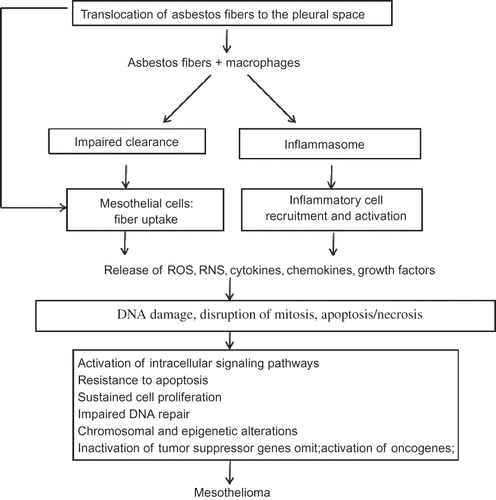
FIGURE 2. Proposed mechanisms for asbestos-induced mesothelioma. Asbestos fibers are thought to lead to mesothelioma via mechanisms as outlined in this algorithm. Asbestos fibers enter the pleural space, where they interact with pleural macrophages and mesothelial cells and induce an influx of inflammatory cells. These early interactions result in release of reactive oxygen and nitrogen species (ROS, RNS), cytokines, and growth factors that may mediate indirect effects on mesothelial cells. The fibers may also act directly on mesothelial cells by inducing DNA damage, interrupting chromosomal segregation, or inducing apoptosis or necrosis. Such direct and indirect actions lead to chronic stimulation and injury of the mesothelium that may proceed over decades by a multistep path to cancer. Key steps in the development of cancer include genetic and epigenetic alterations leading to sustained cell proliferation, resistance to apoptosis, and inactivation of tumor suppressor genes.