Figures & data
TABLE 1. Significant Changes in the Expression of P-gp, CYP3A, and CYP2D Subsequent to Sucralose Ingestion (Delivered in Splenda) Relative to Control (No Sucralose) in Rat
TABLE 2. Representative Organochlorine Drugs Reported to Be Substrates, Inhibitors, or Inducers of CYP isozymes and/or P-gp Using One or More Assay Types
TABLE 2. (Continued)
TABLE 2. (Continued)
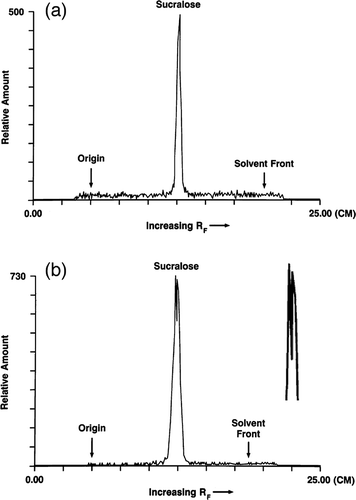
FIGURE 1. Thin-layer radiochromatographic profile of methanolic fecal extracts following both intravenous (iv) and oral administration of 14C-sucralose: (a) 0–24 h fecal sample from a male rat given an iv dose of 14C-sucralose (2 mg/kg); (b) 0–24 h fecal sample from a male rat maintained on a diet containing 30,000 ppm sucralose for 85 wk before receiving an oral dose of 14C-sucralose (100 mg/kg). An enlargement of the peak profile is given to the right. (TLC traces from CitationSims et al., 2000).